Loss-of-function of zebrafish cdt1 causes retarded body growth and underdeveloped gonads resembling human Meier-Gorlin syndrome
- PMID: 37961805
- PMCID: PMC10646402
- DOI: 10.1631/jzus.B2300195
Loss-of-function of zebrafish cdt1 causes retarded body growth and underdeveloped gonads resembling human Meier-Gorlin syndrome
Abstract
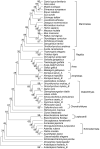
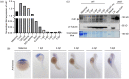
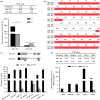
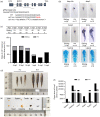
染色质许可和DNA复制因子1(Cdt1)是复制起始许可的主要调控因子,也是组成复制前复合物的核心成员。细胞通过依赖Cdt1的波动水平,且在每个周期中通过调节其总量以确保DNA仅复制一次。Cdt1功能缺陷会造成DNA过度复制,最终导致基因组不稳定。虽然酵母中cdt1和人类Meier-Gorlin综合征(MGS)患者中的CDT1已被广泛研究,但缺乏脊椎动物模型。我们发现在硬骨鱼类分支的几个鲤形目物种(包括斑马鱼)中,Cdt1蛋白在其N末端插入一段其他脊椎动物中没有的独特无序序列。通过分析在cdt1基因中携带移码缺失的遗传性斑马鱼突变体(命名为cdt1zju1 ),我们发现突变胚胎虽然几乎无任何早期胚胎表型异常,但成年突变斑马鱼却表现出侏儒症、生存能力降低的症状,以及性腺发育不全且不育。此外,我们同样发现除转录本cdt1-201外,斑马鱼还存在第二个cdt1转录本——cdt1-202,它是通过跳过外显子2产生,这在其他生物中暂无报道。有意思的是cdt1-202在cdt1-201纯合突变体中显著上调。上述研究结果表明,cdt1-202转录本可能可以补偿cdt1-201在早期发育过程中的功能损失,但不能补偿后期生长,这可支持斑马鱼作为研究人类MGS的遗传模型。
Figures

Similar articles
-
Zebrafish cdc6 hypomorphic mutation causes Meier-Gorlin syndrome-like phenotype.Hum Mol Genet. 2017 Nov 1;26(21):4168-4180. doi: 10.1093/hmg/ddx305. Hum Mol Genet. 2017. PMID: 28985365 Free PMC article.
-
Successful pregnancies in an adult with Meier-Gorlin syndrome harboring biallelic CDT1 variants.Am J Med Genet A. 2021 Mar;185(3):871-876. doi: 10.1002/ajmg.a.62016. Epub 2020 Dec 18. Am J Med Genet A. 2021. PMID: 33338304
-
MCM5: a new actor in the link between DNA replication and Meier-Gorlin syndrome.Eur J Hum Genet. 2017 May;25(5):646-650. doi: 10.1038/ejhg.2017.5. Epub 2017 Feb 15. Eur J Hum Genet. 2017. PMID: 28198391 Free PMC article.
-
Further delineation of CDC45-related Meier-Gorlin syndrome with craniosynostosis and review of literature.Eur J Med Genet. 2020 Feb;63(2):103652. doi: 10.1016/j.ejmg.2019.04.009. Epub 2019 Apr 13. Eur J Med Genet. 2020. PMID: 30986546 Review.
-
Meier-Gorlin syndrome.Orphanet J Rare Dis. 2015 Sep 17;10:114. doi: 10.1186/s13023-015-0322-x. Orphanet J Rare Dis. 2015. PMID: 26381604 Free PMC article. Review.
Cited by
-
Small fish making a big difference: beloved star of environmental toxicology research in the current era.J Zhejiang Univ Sci B. 2025 Jul 15;26(7):613-632. doi: 10.1631/jzus.B2500166. J Zhejiang Univ Sci B. 2025. PMID: 40722242 Free PMC article. Review.
-
Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses.Genes (Basel). 2025 May 27;16(6):637. doi: 10.3390/genes16060637. Genes (Basel). 2025. PMID: 40565529 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

