Nitric Oxide Modulates Ca2+ Leak and Arrhythmias via S-Nitrosylation of CaMKII
- PMID: 37961889
- PMCID: PMC10699507
- DOI: 10.1161/CIRCRESAHA.123.323571
Nitric Oxide Modulates Ca2+ Leak and Arrhythmias via S-Nitrosylation of CaMKII
Abstract
Background: Nitric oxide (NO) has been identified as a signaling molecule generated during β-adrenergic receptor stimulation in the heart. Furthermore, a role for NO in triggering spontaneous Ca2+ release via S-nitrosylation of CaMKIIδ (Ca2+/calmodulin kinase II delta) is emerging. NO donors are routinely used clinically for their cardioprotective effects on the heart, but it is unknown how NO donors modulate the proarrhythmic CaMKII to alter cardiac arrhythmia incidence. We test the role of S-nitrosylation of CaMKIIδ at the Cysteine-273 inhibitory site and cysteine-290 activating site in cardiac Ca2+ handling and arrhythmogenesis before and during β-adrenergic receptor stimulation.
Methods: We measured Ca2+-handling in isolated cardiomyocytes from C57BL/6J wild-type (WT) mice and mice lacking CaMKIIδ expression (CaMKIIδ-KO) or with deletion of the S-nitrosylation site on CaMKIIδ at cysteine-273 or cysteine-290 (CaMKIIδ-C273S and -C290A knock-in mice). Cardiomyocytes were exposed to NO donors, S-nitrosoglutathione (GSNO; 150 μM), sodium nitroprusside (200 μM), and β-adrenergic agonist isoproterenol (100 nmol/L).
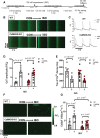
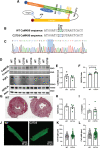
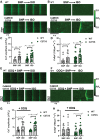
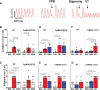
Results: Both WT and CaMKIIδ-KO cardiomyocytes responded to isoproterenol with a full inotropic and lusitropic Ca2+ transient response as well as increased Ca2+ spark frequency. However, the increase in Ca2+ spark frequency was significantly attenuated in CaMKIIδ-KO cardiomyocytes. The protection from isoproterenol-induced Ca2+ sparks and waves was mimicked by GSNO pretreatment in WT cardiomyocytes but lost in CaMKIIδ-C273S cardiomyocytes. When GSNO was applied after isoproterenol, this protection was not observed in WT or CaMKIIδ-C273S but was apparent in CaMKIIδ-C290A. In Langendorff-perfused isolated hearts, GSNO pretreatment limited isoproterenol-induced arrhythmias in WT but not CaMKIIδ-C273S hearts, while GSNO exposure after isoproterenol sustained or exacerbated arrhythmic events.
Conclusions: We conclude that prior S-nitrosylation of CaMKIIδ at cysteine-273 can limit subsequent β-adrenergic receptor-induced arrhythmias, but that S-nitrosylation at cysteine-290 might worsen or sustain β-adrenergic receptor-induced arrhythmias. This has important implications for the administration of NO donors in the clinical setting.
Keywords: calcium; heart; nitric oxide.
Conflict of interest statement
Figures




Similar articles
-
Nitric Oxide modulates spontaneous Ca2+ release and ventricular arrhythmias during β-adrenergic signalling through S-nitrosylation of Calcium/Calmodulin dependent kinase II.bioRxiv [Preprint]. 2023 Aug 24:2023.08.23.554546. doi: 10.1101/2023.08.23.554546. bioRxiv. 2023. PMID: 37662205 Free PMC article. Preprint.
-
Nitrosylation of cardiac CaMKII at Cys290 mediates mechanical afterload-induced increases in Ca2+ transient and Ca2+ sparks.J Physiol. 2022 Nov;600(22):4865-4879. doi: 10.1113/JP283427. Epub 2022 Oct 30. J Physiol. 2022. PMID: 36227145 Free PMC article.
-
CaMKIIδ mediates β-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic β-adrenergic stimulation.J Mol Cell Cardiol. 2015 Aug;85:282-91. doi: 10.1016/j.yjmcc.2015.06.007. Epub 2015 Jun 14. J Mol Cell Cardiol. 2015. PMID: 26080362 Free PMC article.
-
Beta-adrenergic receptor signaling in the heart: role of CaMKII.J Mol Cell Cardiol. 2010 Feb;48(2):322-30. doi: 10.1016/j.yjmcc.2009.10.016. Epub 2009 Oct 31. J Mol Cell Cardiol. 2010. PMID: 19883653 Free PMC article. Review.
-
Protein S-nitrosylation in health and disease: a current perspective.Trends Mol Med. 2009 Sep;15(9):391-404. doi: 10.1016/j.molmed.2009.06.007. Epub 2009 Aug 31. Trends Mol Med. 2009. PMID: 19726230 Free PMC article. Review.
Cited by
-
Beta-Adrenergic Activation of the Inward Rectifier K+ Current Is Mediated by the CaMKII Pathway in Canine Ventricular Cardiomyocytes.Int J Mol Sci. 2024 Oct 29;25(21):11609. doi: 10.3390/ijms252111609. Int J Mol Sci. 2024. PMID: 39519160 Free PMC article.
-
Molecular and cellular neurocardiology in heart disease.J Physiol. 2025 Mar;603(7):1689-1728. doi: 10.1113/JP284739. Epub 2024 May 22. J Physiol. 2025. PMID: 38778747 Review.
-
S-Nitrosylation in Cardiovascular Disorders: The State of the Art.Biomolecules. 2025 Jul 24;15(8):1073. doi: 10.3390/biom15081073. Biomolecules. 2025. PMID: 40867518 Free PMC article. Review.
-
ACAA2 Protects Against Cardiac Dysfunction and Lipid Peroxidation in Renal Insufficiency with the Treatment of S-Nitroso-L-Cysteine.Biomolecules. 2025 Mar 3;15(3):364. doi: 10.3390/biom15030364. Biomolecules. 2025. PMID: 40149900 Free PMC article.
-
An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation.Biomolecules. 2025 May 17;15(5):735. doi: 10.3390/biom15050735. Biomolecules. 2025. PMID: 40427628 Free PMC article.
References
-
- Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. doi: 10.1016/S0021-9258(18)33279-4 - PubMed
-
- Grimm M, Ling H, Willeford A, Pereira L, Gray CB, Erickson JR, Sarma S, Respress JL, Wehrens XH, Bers DM, et al. . CaMKIIdelta mediates beta-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic beta-adrenergic stimulation. J Mol Cell Cardiol. 2015;85:282–291. doi: 10.1016/j.yjmcc.2015.06.007 - PMC - PubMed
-
- Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

