Effect of Mavacamten in Women Compared With Men With Obstructive Hypertrophic Cardiomyopathy: Insights From EXPLORER-HCM
- PMID: 37961906
- PMCID: PMC11006596
- DOI: 10.1161/CIRCULATIONAHA.123.065600
Effect of Mavacamten in Women Compared With Men With Obstructive Hypertrophic Cardiomyopathy: Insights From EXPLORER-HCM
Abstract
Background: Compared with men, women with hypertrophic cardiomyopathy (HCM) have a higher incidence of heart failure and worse outcomes. We investigated baseline clinical and echocardiographic characteristics and response to mavacamten among women compared with men in the EXPLORER-HCM study (Clinical Study to Evaluate Mavacamten [MYK-461] in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy).
Methods: A prespecified post hoc analysis of sex from the blinded, randomized EXPLORER-HCM trial of mavacamten versus placebo in symptomatic patients with obstructive HCM was performed. Baseline characteristics were compared with t tests for continuous variables (expressed as mean values) and χ2 tests for categorical variables. Prespecified primary, secondary, and exploratory end points and echocardiographic measurements from baseline to end of treatment (week 30) were analyzed with ANCOVA for continuous end points and a generalized linear model with binomial distribution for binary end points, with adjustment for each outcome's baseline value, New York Heart Association class, β-blocker use, and ergometer type.
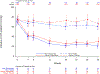
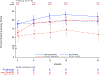
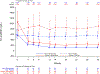
Results: At baseline, women (n=102) were older (62 years versus 56 years; P<0.0001), had lower peak oxygen consumption (16.7 mL·kg-1·min-1 versus 21.3 mL·kg-1·min-1; P<0.0001), were more likely to be assigned New York Heart Association class III (42% versus 17%; P<0.0001), had worse health status (Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score 64 versus 75; P<0.0001), and had higher baseline plasma NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels (1704 ng/L versus 990 ng/L; P=0.004) than men (n=149). After 30 weeks of mavacamten treatment, similar improvements were observed in women and men in the primary composite end point (percentage difference on mavacamten versus placebo, 22% versus 19%, respectively; P=0.759) and in the secondary end points of change in postexercise left ventricular outflow tract gradient (-42.4 mm Hg versus -33.6 mm Hg; P=0.348), change in peak oxygen consumption (1.2 mL·kg-1·min-1 versus 1.6 mL·kg-1·min-1; P=0.633), and percentage achieving ≥1 New York Heart Association class improvement (41% versus 28%; P=0.254). However, women had greater improvement in health status (Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score 14.8 versus 6.1; P=0.026) and in the exploratory end point of NT-proBNP levels (-1322 ng/L versus -649 ng/L; P=0.0008).
Conclusions: Although at baseline women with symptomatic obstructive HCM enrolled in EXPLORER-HCM were older and had worse heart failure and health status than men, treatment with mavacamten resulted in similar improvements in the primary and most secondary EXPLORER-HCM end points and greater improvements in health status and NT-proBNP.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03470545.
Keywords: clinical study; heart failure.
Conflict of interest statement
Figures
References
-
- Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e533–e557. - PubMed
-
- Maron BJ. Clinical Course and Management of Hypertrophic Cardiomyopathy. N Engl J Med. 2018;379:655–668. - PubMed
-
- Semsarian C, Ingles J, Maron MS and Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. - PubMed
-
- Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R and Olivotto I. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy in the United States. Am J Cardiol. 2016;117:1651–1654. - PubMed
-
- Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F and Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

