An intronic RNA element modulates Factor VIII exon-16 splicing
- PMID: 37962303
- PMCID: PMC10783525
- DOI: 10.1093/nar/gkad1034
An intronic RNA element modulates Factor VIII exon-16 splicing
Abstract
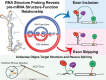
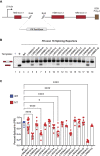
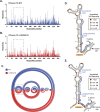
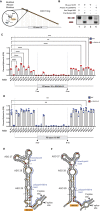
Pathogenic variants in the human Factor VIII (F8) gene cause Hemophilia A (HA). Here, we investigated the impact of 97 HA-causing single-nucleotide variants on the splicing of 11 exons from F8. For the majority of F8 exons, splicing was insensitive to the presence of HA-causing variants. However, splicing of several exons, including exon-16, was impacted by variants predicted to alter exonic splicing regulatory sequences. Using exon-16 as a model, we investigated the structure-function relationship of HA-causing variants on splicing. Intriguingly, RNA chemical probing analyses revealed a three-way junction structure at the 3'-end of intron-15 (TWJ-3-15) capable of sequestering the polypyrimidine tract. We discovered antisense oligonucleotides (ASOs) targeting TWJ-3-15 partially rescue splicing-deficient exon-16 variants by increasing accessibility of the polypyrimidine tract. The apical stem loop region of TWJ-3-15 also contains two hnRNPA1-dependent intronic splicing silencers (ISSs). ASOs blocking these ISSs also partially rescued splicing. When used in combination, ASOs targeting both the ISSs and the region sequestering the polypyrimidine tract, fully rescue pre-mRNA splicing of multiple HA-linked variants of exon-16. Together, our data reveal a putative RNA structure that sensitizes F8 exon-16 to aberrant splicing.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
OpenASO: RNA Rescue - designing splice-modulating antisense oligonucleotides through community science.bioRxiv [Preprint]. 2024 Oct 16:2024.10.15.618608. doi: 10.1101/2024.10.15.618608. bioRxiv. 2024. Update in: RNA. 2025 Jul 16;31(8):1091-1102. doi: 10.1261/rna.080288.124. PMID: 39463988 Free PMC article. Updated. Preprint.
-
OpenASO: RNA Rescue-designing splice-modulating antisense oligonucleotides through community science.RNA. 2025 Jul 16;31(8):1091-1102. doi: 10.1261/rna.080288.124. RNA. 2025. PMID: 40425312
-
Rescue of blood coagulation Factor VIII exon-16 mis-splicing by antisense oligonucleotides.bioRxiv [Preprint]. 2023 Apr 1:2023.03.31.535160. doi: 10.1101/2023.03.31.535160. bioRxiv. 2023. PMID: 37034721 Free PMC article. Preprint.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
A novel splice site variant in DEGS1 leads to aberrant splicing and loss of DEGS1 enzyme activity, a VUS resolved.medRxiv [Preprint]. 2025 Apr 11:2025.04.04.25325118. doi: 10.1101/2025.04.04.25325118. medRxiv. 2025. PMID: 40297416 Free PMC article. Preprint.
-
OpenASO: RNA Rescue - designing splice-modulating antisense oligonucleotides through community science.bioRxiv [Preprint]. 2024 Oct 16:2024.10.15.618608. doi: 10.1101/2024.10.15.618608. bioRxiv. 2024. Update in: RNA. 2025 Jul 16;31(8):1091-1102. doi: 10.1261/rna.080288.124. PMID: 39463988 Free PMC article. Updated. Preprint.
-
RNA structure in alternative splicing regulation: from mechanism to therapy.Acta Biochim Biophys Sin (Shanghai). 2024 Jul 22;57(1):3-21. doi: 10.3724/abbs.2024119. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 39034824 Free PMC article. Review.
-
All exons are not created equal-exon vulnerability determines the effect of exonic mutations on splicing.Nucleic Acids Res. 2024 May 8;52(8):4588-4603. doi: 10.1093/nar/gkae077. Nucleic Acids Res. 2024. PMID: 38324470 Free PMC article.
References
-
- Chow L.T., Gelinas R.E., Broker T.R., Roberts R.J.. An amazing sequence arrangement at the 5’ ends of adenovirus 2 messenger RNA. Cell. 1977; 12:1–8. - PubMed
-
- Konarska M.M., Sharp P.A.. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987; 49:763–774. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

