Humanization reveals pervasive incompatibility of yeast and human kinetochore components
- PMID: 37962556
- PMCID: PMC10755175
- DOI: 10.1093/g3journal/jkad260
Humanization reveals pervasive incompatibility of yeast and human kinetochore components
Abstract
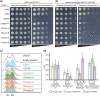
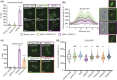
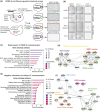
Kinetochores assemble on centromeres to drive chromosome segregation in eukaryotic cells. Humans and budding yeast share most of the structural subunits of the kinetochore, whereas protein sequences have diverged considerably. The conserved centromeric histone H3 variant, CenH3 (CENP-A in humans and Cse4 in budding yeast), marks the site for kinetochore assembly in most species. A previous effort to complement Cse4 in yeast with human CENP-A was unsuccessful; however, co-complementation with the human core nucleosome was not attempted. Previously, our lab successfully humanized the core nucleosome in yeast; however, this severely affected cellular growth. We hypothesized that yeast Cse4 is incompatible with humanized nucleosomes and that the kinetochore represented a limiting factor for efficient histone humanization. Thus, we argued that including the human CENP-A or a Cse4-CENP-A chimera might improve histone humanization and facilitate kinetochore function in humanized yeast. The opposite was true: CENP-A expression reduced histone humanization efficiency, was toxic to yeast, and disrupted cell cycle progression and kinetochore function in wild-type (WT) cells. Suppressors of CENP-A toxicity included gene deletions of subunits of 3 conserved chromatin remodeling complexes, highlighting their role in CenH3 chromatin positioning. Finally, we attempted to complement the subunits of the NDC80 kinetochore complex, individually and in combination, without success, in contrast to a previous study indicating complementation by the human NDC80/HEC1 gene. Our results suggest that limited protein sequence similarity between yeast and human components in this very complex structure leads to failure of complementation.
Keywords: CENP-A; centromere; histone variant; humanization; kinetochore; yeast.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest J.D.B. is a founder and director of CDI Labs, Inc.; a founder of and consultant to Neochromosome, Inc.; and a founder, SAB member of, and consultant to ReOpen Diagnostics, LLC, and serves or served on the scientific advisory board of the following: LogoMix Inc.; Modern Meadow Inc.; ROME Therapeutics, Inc.; Sample6 Inc.; Sangamo, Inc.; Tessera Therapeutics Inc., and the Wyss Institute. G.O. and M.A.B.H. declare no competing interests.
Figures


Similar articles
-
Methylation of CENP-A/Cse4 on arginine 143 and lysine 131 regulates kinetochore stability in yeast.Genetics. 2023 Apr 6;223(4):iyad028. doi: 10.1093/genetics/iyad028. Genetics. 2023. PMID: 36810679 Free PMC article.
-
A Genome-Wide Screen Reveals a Role for the HIR Histone Chaperone Complex in Preventing Mislocalization of Budding Yeast CENP-A.Genetics. 2018 Sep;210(1):203-218. doi: 10.1534/genetics.118.301305. Epub 2018 Jul 16. Genetics. 2018. PMID: 30012561 Free PMC article.
-
Histone H4 Facilitates the Proteolysis of the Budding Yeast CENP-ACse4 Centromeric Histone Variant.Genetics. 2017 Jan;205(1):113-124. doi: 10.1534/genetics.116.194027. Epub 2016 Oct 28. Genetics. 2017. PMID: 27794026 Free PMC article.
-
Protein kinases in mitotic phosphorylation of budding yeast CENP-A.Curr Genet. 2019 Dec;65(6):1325-1332. doi: 10.1007/s00294-019-00997-5. Epub 2019 May 22. Curr Genet. 2019. PMID: 31119371 Review.
-
Conserved and divergent mechanisms of inner kinetochore assembly onto centromeric chromatin.Curr Opin Struct Biol. 2023 Aug;81:102638. doi: 10.1016/j.sbi.2023.102638. Epub 2023 Jun 20. Curr Opin Struct Biol. 2023. PMID: 37343495 Review.
Cited by
-
A role for the kinetochore protein, NUF2, in ribosome biogenesis.Mol Biol Cell. 2025 Feb 1;36(2):ar16. doi: 10.1091/mbc.E24-08-0337. Epub 2024 Dec 20. Mol Biol Cell. 2025. PMID: 39705402 Free PMC article.
References
-
- Abdullah M, Greco BM, Laurent JM, Garge RK, Boutz DR, Vandeloo M, Marcotte EM, Kachroo AH. 2023. Rapid, scalable, combinatorial genome engineering by marker-less enrichment and recombination of genetically engineered loci in yeast. Cell Rep Methods. 3(5):100464. doi:10.1016/J.CRMETH.2023.100464. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
