A partial human LCK defect causes a T cell immunodeficiency with intestinal inflammation
- PMID: 37962568
- PMCID: PMC10644909
- DOI: 10.1084/jem.20230927
A partial human LCK defect causes a T cell immunodeficiency with intestinal inflammation
Abstract
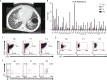
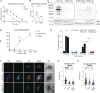
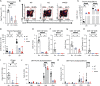
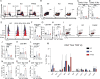
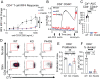
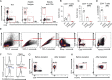
Lymphocyte-specific protein tyrosine kinase (LCK) is essential for T cell antigen receptor (TCR)-mediated signal transduction. Here, we report two siblings homozygous for a novel LCK variant (c.1318C>T; P440S) characterized by T cell lymphopenia with skewed memory phenotype, infant-onset recurrent infections, failure to thrive, and protracted diarrhea. The patients' T cells show residual TCR signal transduction and proliferation following anti-CD3/CD28 and phytohemagglutinin (PHA) stimulation. We demonstrate in mouse models that complete (Lck-/-) versus partial (LckP440S/P440S) loss-of-function LCK causes disease with differing phenotypes. While both Lck-/- and LckP440S/P440S mice exhibit arrested thymic T cell development and profound T cell lymphopenia, only LckP440S/P440S mice show residual T cell proliferation, cytokine production, and intestinal inflammation. Furthermore, the intestinal disease in the LckP440S/P440S mice is prevented by CD4+ T cell depletion or regulatory T cell transfer. These findings demonstrate that P440S LCK spares sufficient T cell function to allow the maturation of some conventional T cells but not regulatory T cells-leading to intestinal inflammation.
© 2023 Lui et al.
Conflict of interest statement
Disclosures: M. Hoenig reported personal fees from Octapharma (scientific board) outside the submitted work. J. Capera reported grants from Cue Biopharma during the conduct of the study. M.L. Dustin reported grants from Cue Biopharma during the conduct of the study. No other disclosures were reported.
Figures








Similar articles
-
Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency.J Allergy Clin Immunol. 2012 Nov;130(5):1144-1152.e11. doi: 10.1016/j.jaci.2012.07.029. Epub 2012 Sep 15. J Allergy Clin Immunol. 2012. PMID: 22985903
-
Differentiation and activation of human CD4 T cells is associated with a gradual loss of myelin and lymphocyte protein.Eur J Immunol. 2021 Apr;51(4):848-863. doi: 10.1002/eji.202048603. Epub 2021 Jan 25. Eur J Immunol. 2021. PMID: 33345332 Free PMC article.
-
Defect of lck in a patient with common variable immunodeficiency.Int J Mol Med. 2001 Jun;7(6):609-14. doi: 10.3892/ijmm.7.6.609. Int J Mol Med. 2001. PMID: 11351273
-
A Novel Biallelic LCK Variant Resulting in Profound T-Cell Immune Deficiency and Review of the Literature.J Clin Immunol. 2023 Dec 15;44(1):1. doi: 10.1007/s10875-023-01602-8. J Clin Immunol. 2023. PMID: 38100037 Free PMC article. Review.
-
Consequences of a mutation in the UNC119 gene for T cell function in idiopathic CD4 lymphopenia.Curr Allergy Asthma Rep. 2012 Oct;12(5):396-401. doi: 10.1007/s11882-012-0281-4. Curr Allergy Asthma Rep. 2012. PMID: 22729960 Review.
Cited by
-
Lck Function and Modulation: Immune Cytotoxic Response and Tumor Treatment More Than a Simple Event.Cancers (Basel). 2024 Jul 24;16(15):2630. doi: 10.3390/cancers16152630. Cancers (Basel). 2024. PMID: 39123358 Free PMC article. Review.
-
Helper T cell immunity in humans with inherited CD4 deficiency.J Exp Med. 2024 May 6;221(5):e20231044. doi: 10.1084/jem.20231044. Epub 2024 Apr 1. J Exp Med. 2024. PMID: 38557723 Free PMC article.
-
SMAD4 Regulates the Expression of LCK Affecting Chimeric Antigen Receptor-T Cells Proliferation Through PI3K/Akt Signaling Pathway.J Cell Physiol. 2025 Jan;240(1):e31520. doi: 10.1002/jcp.31520. J Cell Physiol. 2025. PMID: 39763264 Free PMC article.
-
Disrupting the balance between activating and inhibitory receptors of γδT cells for effective cancer immunotherapy.Nat Rev Cancer. 2025 Aug;25(8):590-612. doi: 10.1038/s41568-025-00830-x. Epub 2025 Jun 2. Nat Rev Cancer. 2025. PMID: 40456901 Review.
-
Immunopathological and microbial signatures of inflammatory bowel disease in partial RAG deficiency.J Exp Med. 2025 Aug 4;222(8):e20241993. doi: 10.1084/jem.20241993. Epub 2025 May 2. J Exp Med. 2025. PMID: 40314722
References
-
- Arnaiz-Villena, A., Timon M., Corell A., Perez-Aciego P., Martin-Villa J.M., and Regueiro J.R.. 1992. Brief report: Primary immunodeficiency caused by mutations in the gene encoding the CD3-gamma subunit of the T-lymphocyte receptor. N. Engl. J. Med. 327:529–533. 10.1056/NEJM199208203270805 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

