The evidence-based role of catecholaminergic PET tracers in Neuroblastoma. A systematic review and a head-to-head comparison with mIBG scintigraphy
- PMID: 37962616
- PMCID: PMC10796700
- DOI: 10.1007/s00259-023-06486-9
The evidence-based role of catecholaminergic PET tracers in Neuroblastoma. A systematic review and a head-to-head comparison with mIBG scintigraphy
Abstract
Background: Molecular imaging is pivotal in staging and response assessment of children with neuroblastoma (NB). [123I]-metaiodobenzylguanidine (mIBG) is the standard imaging method; however, it is characterised by low spatial resolution, time-consuming acquisition procedures and difficult interpretation. Many PET catecholaminergic radiotracers have been proposed as a replacement for [123I]-mIBG, however they have not yet made it into clinical practice. We aimed to review the available literature comparing head-to-head [123I]-mIBG with the most common PET catecholaminergic radiopharmaceuticals.
Methods: We searched the PubMed database for studies performing a head-to-head comparison between [123I]-mIBG and PET radiopharmaceuticals including meta-hydroxyephedrine ([11C]C-HED), 18F-18F-3,4-dihydroxyphenylalanine ([18F]DOPA) [124I]mIBG and Meta-[18F]fluorobenzylguanidine ([18F]mFBG). Review articles, preclinical studies, small case series (< 5 subjects), case reports, and articles not in English were excluded. From each study, the following characteristics were extracted: bibliographic information, technical parameters, and the sensitivity of the procedure according to a patient-based analysis (PBA) and a lesion-based analysis (LBA).
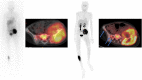
Results: Ten studies were selected: two regarding [11C]C-HED, four [18F]DOPA, one [124I]mIBG, and three [18F]mFBG. These studies included 181 patients (range 5-46). For the PBA, the superiority of the PET method was reported in two out of ten studies (both using [18F]DOPA). For LBA, PET detected significantly more lesions than scintigraphy in seven out of ten studies.
Conclusions: PET/CT using catecholaminergic tracers shows superior diagnostic performance than mIBG scintigraphy. However, it is still unknown if such superiority can influence clinical decision-making. Nonetheless, the PET examination appears promising for clinical practice as it offers faster image acquisition, less need for sedation, and a single-day examination.
Keywords: Catecholamine; Guideline; Neuroblastoma; PET-CT; Paediatric PET; [124I]MIBG, 18F-MFBG, 11C-HED; [18F]F-DOPA.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J Clin Oncol. 2009;27:1007–13. 10.1200/JCO.2007.13.8925. - PMC - PubMed
-
- Bar-Sever Z, Biassoni L, Shulkin B, Kong G, Hofman MS, Lopci E, et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur J Nucl Med Mol Imaging. 2018;45:2009–24. 10.1007/s00259-018-4070-8. - PubMed
-
- Ladenstein R, Lambert B, Pötschger U, Castellani MR, Lewington V, Bar-Sever Z, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging. 2018;45:292–305. 10.1007/s00259-017-3829-7. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

