A fluorogenic micrococcal nuclease-based probe for fast detection and optical imaging of Staphylococcus aureus in prosthetic joint and fracture-related infections
- PMID: 37962617
- PMCID: PMC11300479
- DOI: 10.1007/s00259-023-06499-4
A fluorogenic micrococcal nuclease-based probe for fast detection and optical imaging of Staphylococcus aureus in prosthetic joint and fracture-related infections
Erratum in
-
Correction to: A fluorogenic micrococcal nuclease‑based probe for fast detection and optical imaging of Staphylococcus aureus in prosthetic joint and fracture‑related infections.Eur J Nucl Med Mol Imaging. 2024 Aug;51(10):3149. doi: 10.1007/s00259-023-06538-0. Eur J Nucl Med Mol Imaging. 2024. PMID: 37994958 Free PMC article. No abstract available.
Abstract
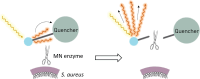
Purpose: Staphylococcus aureus is the most common and impactful multi-drug resistant pathogen implicated in (periprosthetic) joint infections (PJI) and fracture-related infections (FRI). Therefore, the present proof-of-principle study was aimed at the rapid detection of S. aureus in synovial fluids and biofilms on extracted osteosynthesis materials through bacteria-targeted fluorescence imaging with the 'smart-activatable' DNA-based AttoPolyT probe. This fluorogenic oligonucleotide probe yields large fluorescence increases upon cleavage by micrococcal nuclease, an enzyme secreted by S. aureus.
Methods: Synovial fluids from patients with suspected PJI and extracted osteosynthesis materials from trauma patients with suspected FRI were inspected for S. aureus nuclease activity with the AttoPolyT probe. Biofilms on osteosynthesis materials were imaged with the AttoPolyT probe and a vancomycin-IRDye800CW conjugate (vanco-800CW) specific for Gram-positive bacteria.
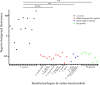
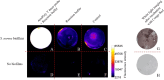
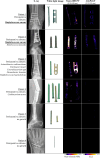
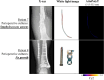
Results: 38 synovial fluid samples were collected and analyzed. Significantly higher fluorescence levels were measured for S. aureus-positive samples compared to, respectively, other Gram-positive bacterial pathogens (p < 0.0001), Gram-negative bacterial pathogens (p = 0.0038) and non-infected samples (p = 0.0030), allowing a diagnosis of S. aureus-associated PJI within 2 h. Importantly, S. aureus-associated biofilms on extracted osteosynthesis materials from patients with FRI were accurately imaged with the AttoPolyT probe, allowing their correct distinction from biofilms formed by other Gram-positive bacteria detected with vanco-800CW within 15 min.
Conclusion: The present study highlights the potential clinical value of the AttoPolyT probe for fast and accurate detection of S. aureus infection in synovial fluids and biofilms on extracted osteosynthesis materials.
Keywords: Staphylococcus aureus; Fracture-related infection; Infection imaging; Micrococcal nuclease; Optical probe; Orthopedic infection; Periprosthetic joint infection.
© 2023. The Author(s).
Conflict of interest statement
James O. McNamara is the founder and CEO of Nuclease Probe Technologies. The other authors have no conflicts of interest to disclose.
Figures
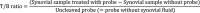
Similar articles
-
Synovial Fluid-Induced Aggregation Occurs across Staphylococcus aureus Clinical Isolates and is Mechanistically Independent of Attached Biofilm Formation.Microbiol Spectr. 2021 Oct 31;9(2):e0026721. doi: 10.1128/Spectrum.00267-21. Epub 2021 Sep 15. Microbiol Spectr. 2021. PMID: 34523997 Free PMC article.
-
Bacteria-targeted fluorescence imaging of extracted osteosynthesis devices for rapid visualization of fracture-related infections.Eur J Nucl Med Mol Imaging. 2022 Jun;49(7):2276-2289. doi: 10.1007/s00259-022-05695-y. Epub 2022 Jan 26. Eur J Nucl Med Mol Imaging. 2022. PMID: 35079847 Free PMC article.
-
The smart activatable P2&3TT probe allows accurate, fast, and highly sensitive detection of Staphylococcus aureus in clinical blood culture samples.Sci Rep. 2020 Nov 5;10(1):19216. doi: 10.1038/s41598-020-76254-4. Sci Rep. 2020. PMID: 33154413 Free PMC article.
-
Ultrasensitive detection of micrococcal nuclease activity and Staphylococcus aureus contamination using optical biosensor technology-A review.Talanta. 2021 May 1;226:122168. doi: 10.1016/j.talanta.2021.122168. Epub 2021 Jan 30. Talanta. 2021. PMID: 33676710 Review.
-
Risk of orthopaedic implant infection during bacteraemia.APMIS. 2025 Jan;133(1):e13482. doi: 10.1111/apm.13482. Epub 2024 Oct 24. APMIS. 2025. PMID: 39444284 Review.
Cited by
-
Functionalized zeolite regulates bone metabolic microenvironment.Mater Today Bio. 2025 Feb 5;31:101558. doi: 10.1016/j.mtbio.2025.101558. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40034985 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

