Radiation exposure assessment of nuclear medicine staff administering [177Lu]Lu-DOTA-TATE with active and passive dosimetry
- PMID: 37962683
- PMCID: PMC10645926
- DOI: 10.1186/s40658-023-00592-1
Radiation exposure assessment of nuclear medicine staff administering [177Lu]Lu-DOTA-TATE with active and passive dosimetry
Abstract
Background: The use of lutetium-177 (177Lu)-based radiopharmaceuticals in peptide receptor nuclear therapy is increasing, but so is the number of nuclear medicine workers exposed to higher levels of radiation. In recent years, [177Lu]Lu-DOTA-TATE has begun to be widely used for the treatment of neuroendocrine tumours. However, there are few studies evaluating the occupational radiation exposure during its administration, and there are still some challenges that can result in higher doses to the staff, such as a lack of trained personnel or fully standardised procedures. In response, this study aims to provide a comprehensive analysis of occupational doses to the staff involved in the administration of [177Lu]Lu-DOTA-TATE.
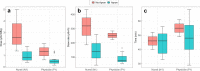
Results: A total of 32 administrations of [177Lu]Lu-DOTA-TATE (7.4 GBq/session) carried out by a physician and a nurse, were studied. In total, two physicians and four nurses were independently monitored with cumulative (passive) and/or real-time (active) dosemeters. Extremity, eye lens and whole-body doses were evaluated in terms of the dosimetric quantities Hp(0.07), Hp(3) and Hp(10), respectively. It was obtained that lead aprons reduced dose rates and whole-body doses by 71% and 69% for the physicians, respectively, and by 56% and 68% for the nurses. On average, normalised Hp(10) values of 0.65 ± 0.18 µSv/GBq were obtained with active dosimetry, which is generally consistent with passive dosemeters. For physicians, the median of the maximum normalised Hp(0.07) values was 41.5 µSv/GBq on the non-dominant hand and 45.2 µSv/GBq on the dominant hand. For nurses 15.4 µSv/GBq on the non-dominant and 13.9 µSv/GBq on the dominant hand. The ratio or correction factor between the maximum dose measured on the hand and the dose measured on the base of the middle/ring finger of the non-dominant hand resulted in a factor of 5/6 for the physicians and 3/4 for the nurses. Finally, maximum normalised Hp(3) doses resulted in 2.02 µSv/GBq for physicians and 1.76 µSv/GBq for nurses.
Conclusions: If appropriate safety measures are taken, the administration of [177Lu]Lu-DOTA-TATE is a safe procedure for workers. However, regular monitoring is recommended to ensure that the annual dose limits are not exceeded.
Keywords: Equivalent dose; Nuclear medicine; Occupational exposure; [177Lu]Lu-DOTA-TATE.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Kaur CD, Mishra KK, Sahu A, Panik R, Kashyap P, Mishra SP, Kumar A. Theranostics: new era in nuclear medicine and radiopharmaceuticals. In: Medical isotopes. IntechOpen London, UK; 2020.
-
- Maughan NM, Kim H, Hao Y, Unangst S, Roach MC, Garcia-Ramirez JL, et al. Initial experience and lessons learned with implementing Lutetium-177-dotatate radiopharmaceutical therapy in a radiation oncology-based program. Brachytherapy. 2021;20(1):237–247. doi: 10.1016/j.brachy.2020.07.004. - DOI - PubMed
-
- EMA. Authorization details for Lutathera in Europe. 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

