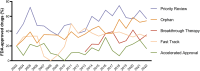
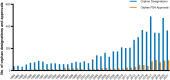
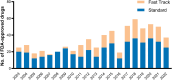
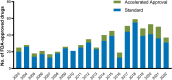
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
- PMID: 37962724
- PMCID: PMC11283430
- DOI: 10.1007/s10198-023-01639-x
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
Abstract
Background: Over the past decades, US Congress enabled the US Food and Drug Administration (FDA) to facilitate and expedite drug development for serious conditions filling unmet medical needs with five special designations and review pathways: orphan, fast track, accelerated approval, priority review, and breakthrough therapy.
Objectives: This study reviews the FDA's five special designations for drug development regarding their safety, efficacy/clinical benefit, clinical trials, innovation, economic incentives, development timelines, and price.
Methods: We conducted a keyword search to identify studies analyzing the impact of the FDA's special designations (orphan, fast track, accelerated approval, priority review, and breakthrough therapy) on the safety, efficacy/clinical benefit, trials, innovativeness, economic incentives, development times, and pricing of new drugs. Results were summarized in a narrative overview.
Results: Expedited approval reduces new drugs' time to market. However, faster drug development and regulatory review are associated with more unrecognized adverse events and post-marketing safety revisions. Clinical trials supporting special FDA approvals frequently use small, non-randomized, open-label designs. Required post-approval trials to monitor unknown adverse events are often delayed or not even initiated. Evidence suggests that drugs approved under special review pathways, marketed as "breakthroughs", are more innovative and deliver a higher clinical benefit than those receiving standard FDA approval. Special designations are an economically viable strategy for investors and pharmaceutical companies to develop drugs for rare diseases with unmet medical needs, due to financial incentives, expedited development timelines, higher clinical trial success rates, alongside greater prices. Nonetheless, patients, physicians, and insurers are concerned about spending money on drugs without a proven benefit or even on drugs that turn out to be ineffective. While European countries established performance- and financial-based managed entry agreements to account for this uncertainty in clinical trial evidence and cost-effectiveness, the pricing and reimbursement of these drugs remain largely unregulated in the US.
Conclusion: Special FDA designations shorten clinical development and FDA approval times for new drugs treating rare and severe diseases with unmet medical needs. Special-designated drugs offer a greater clinical benefit to patients. However, physicians, patients, and insurers must be aware that special-designated drugs are often approved based on non-robust trials, associated with more unrecognized side effects, and sold for higher prices.
Keywords: Accelerated approval; Breakthrough therapy; Clinical trial; Drug development; Drug price; Efficacy; European medicines agency; Fast track; Healthcare policy; Innovation; Orphan designation; Pharmaceutical policy; Priority review; Safety; Special designation; US food and drug administration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Clinical benefit, development, innovation, trials, epidemiology, and price for cancer drugs and indications with multiple special FDA designations.J Natl Cancer Inst. 2024 Feb 8;116(2):216-229. doi: 10.1093/jnci/djad212. J Natl Cancer Inst. 2024. PMID: 37824202
-
Ethical imperatives of timely access to orphan drugs: is possible to reconcile economic incentives and patients' health needs?Orphanet J Rare Dis. 2017 Jan 5;12(1):1. doi: 10.1186/s13023-016-0551-7. Orphanet J Rare Dis. 2017. PMID: 28057032 Free PMC article.
-
Initial and supplementary indication approval of new targeted cancer drugs by the FDA, EMA, Health Canada, and TGA.Invest New Drugs. 2022 Aug;40(4):798-809. doi: 10.1007/s10637-022-01227-5. Epub 2022 Apr 7. Invest New Drugs. 2022. PMID: 35389145 Free PMC article.
-
The US orphan drug programme 1983-1995.Pharmacoeconomics. 1997 Sep;12(3):312-26. doi: 10.2165/00019053-199712030-00004. Pharmacoeconomics. 1997. PMID: 10170457 Review.
-
An overview of cancer drugs approved through expedited approval programs and orphan medicine designation globally between 2011 and 2020.Drug Discov Today. 2022 May;27(5):1236-1250. doi: 10.1016/j.drudis.2021.12.021. Epub 2021 Dec 29. Drug Discov Today. 2022. PMID: 34971818 Review.
Cited by
-
Launch and Post-Launch Prices of Injectable Cancer Drugs in the US: Clinical Benefit, Innovation, Epidemiology, and Competition.Pharmacoeconomics. 2024 Jan;42(1):117-131. doi: 10.1007/s40273-023-01320-4. Epub 2023 Oct 19. Pharmacoeconomics. 2024. PMID: 37855850 Free PMC article.
-
The priority review voucher: a misconceived quid pro quo.BMJ Glob Health. 2024 Dec 3;9(12):e015933. doi: 10.1136/bmjgh-2024-015933. BMJ Glob Health. 2024. PMID: 39631790 Free PMC article. No abstract available.
-
A New Era of Management of Von Hippel-Lindau Disease-Associated Tumors With Belzutifan.Clin J Oncol Nurs. 2025 Jan 17;29(1):25-29. doi: 10.1188/25.CJON.25-29. Clin J Oncol Nurs. 2025. PMID: 39933087 Free PMC article.
-
Are We Losing the Final Fight against Cancer?Cancers (Basel). 2024 Jan 19;16(2):421. doi: 10.3390/cancers16020421. Cancers (Basel). 2024. PMID: 38275862 Free PMC article.
-
Use of Basket Trials to Solve Sleep Problems in Patients with Rare Diseases.Clocks Sleep. 2024 Nov 5;6(4):656-667. doi: 10.3390/clockssleep6040044. Clocks Sleep. 2024. PMID: 39584973 Free PMC article.
References
-
- Vreman, R.A., Heikkinen, I., Schuurman, A., Sapede, C., Garcia, J.L., Hedberg, N., Athanasiou, D., Grueger, J., Leufkens, H.G.M., Goettsch, W.G.: Unmet medical need: an introduction to definitions and stakeholder perceptions. Value Health 22, 1275–1282 (2019) 10.1016/j.jval.2019.07.007 - DOI - PubMed
-
- FDA.: Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review. In: US Food Drug Adm. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-... (2018). Accessed 28 Dec 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

