Early mobilisation in critically ill COVID-19 patients: a subanalysis of the ESICM-initiated UNITE-COVID observational study
- PMID: 37962748
- PMCID: PMC10645963
- DOI: 10.1186/s13613-023-01201-1
Early mobilisation in critically ill COVID-19 patients: a subanalysis of the ESICM-initiated UNITE-COVID observational study
Abstract
Background: Early mobilisation (EM) is an intervention that may improve the outcome of critically ill patients. There is limited data on EM in COVID-19 patients and its use during the first pandemic wave.
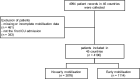
Methods: This is a pre-planned subanalysis of the ESICM UNITE-COVID, an international multicenter observational study involving critically ill COVID-19 patients in the ICU between February 15th and May 15th, 2020. We analysed variables associated with the initiation of EM (within 72 h of ICU admission) and explored the impact of EM on mortality, ICU and hospital length of stay, as well as discharge location. Statistical analyses were done using (generalised) linear mixed-effect models and ANOVAs.
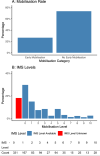
Results: Mobilisation data from 4190 patients from 280 ICUs in 45 countries were analysed. 1114 (26.6%) of these patients received mobilisation within 72 h after ICU admission; 3076 (73.4%) did not. In our analysis of factors associated with EM, mechanical ventilation at admission (OR 0.29; 95% CI 0.25, 0.35; p = 0.001), higher age (OR 0.99; 95% CI 0.98, 1.00; p ≤ 0.001), pre-existing asthma (OR 0.84; 95% CI 0.73, 0.98; p = 0.028), and pre-existing kidney disease (OR 0.84; 95% CI 0.71, 0.99; p = 0.036) were negatively associated with the initiation of EM. EM was associated with a higher chance of being discharged home (OR 1.31; 95% CI 1.08, 1.58; p = 0.007) but was not associated with length of stay in ICU (adj. difference 0.91 days; 95% CI - 0.47, 1.37, p = 0.34) and hospital (adj. difference 1.4 days; 95% CI - 0.62, 2.35, p = 0.24) or mortality (OR 0.88; 95% CI 0.7, 1.09, p = 0.24) when adjusted for covariates.
Conclusions: Our findings demonstrate that a quarter of COVID-19 patients received EM. There was no association found between EM in COVID-19 patients' ICU and hospital length of stay or mortality. However, EM in COVID-19 patients was associated with increased odds of being discharged home rather than to a care facility. Trial registration ClinicalTrials.gov: NCT04836065 (retrospectively registered April 8th 2021).
Keywords: Bed rest; COVID-19; Critical care; Early ambulation; Intensive care units; Mobilisation; Physical therapy specialty; SARS-CoV-2.
© 2023. The Author(s).
Conflict of interest statement
SJS received grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), grants from the Innovationsfond of The Federal Joint Committee (G-BA), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organisers) in the field of anesthesiology and intensive care medicine, outside the submitted work. Dr. Schaller holds stocks in small amounts from Alphabeth Inc., Bayer AG and Siemens AG; these holdings have not affected any decisions regarding his research or this study. GC reports research grants and personal fees as a Speakers' Bureau Member and Advisory Board Member from Integra, Neuroptics, Biogen, Idorsia and Invex Therapeutics all not related with this study. MO has received lecture fees and research funding from Baxter, Fresenius Medical and Biomerieux. JDW is supported by a Senior Clinical Investigator Grant from the Flanders Research Foundation (ref. 1881020N). JDW has consulted for Pfizer, MSD, ThermoFisher (honoraria paid to institution). All other authors declare no competing interests.
Figures

References
-
- Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

