Industry Involvement and Transparency in the Most Cited Clinical Trials, 2019-2022
- PMID: 37962883
- PMCID: PMC10646728
- DOI: 10.1001/jamanetworkopen.2023.43425
Industry Involvement and Transparency in the Most Cited Clinical Trials, 2019-2022
Abstract
Importance: Industry involvement is prominent in influential clinical trials, and commitments to transparency of trials are highly variable.
Objective: To evaluate the modes of industry involvement and the transparency features of the most cited recent clinical trials across medicine.
Design, setting, and participants: This cross-sectional study was a meta-research assessment including randomized and nonrandomized clinical trials published in 2019 or later. The 600 trials of any type of disease or setting that attracted highest number of citations in Scopus as of December 2022 were selected for analysis. Data were analyzed from March to September 2023.
Main outcomes and measures: Outcomes of interest were industry involvement (sponsor, author, and analyst) and transparency (protocols, statistical analysis plans, and data and code availability).
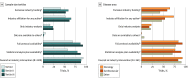
Results: Among 600 trials with a median (IQR) sample size of 415 (124-1046) participants assessed, 409 (68.2%) had industry funding and 303 (50.5%) were exclusively industry-funded. A total of 354 trials (59.0%) had industry authors, with 280 trials (46.6%) involving industry analysts and 125 trials (20.8%) analyzed exclusively by industry analysts. Among industry-funded trials, 364 (89.0%) reached conclusions favoring the sponsor. Most trials (478 trials [79.7%]) provided a data availability statement, and most indicated intention to share the data, but only 16 trials (2.7%) had data already readily available to others. More than three-quarters of trials had full protocols (482 trials [82.0%]) or statistical analysis plans (446 trials [74.3%]) available, but only 27 trials (4.5%) explicitly mentioned sharing analysis code (8 readily available; 19 on request). Randomized trials were more likely than nonrandomized studies to involve only industry analysts (107 trials [22.9%] vs 18 trials [13.6%]; P = .02) and to have full protocols (405 studies [86.5%] vs 87 studies [65.9%]; P < .001) and statistical analysis plans (373 studies [79.7%] vs 73 studies [55.3%]; P < .001) available. Almost all nonrandomized industry-funded studies (90 of 92 studies [97.8%]) favored the sponsor. Among industry-funded trials, exclusive industry funding (odds ratio, 2.9; 95% CI, 1.5-5.4) and industry-affiliated authors (odds ratio, 2.9; 95% CI, 1.5-5.6) were associated with favorable conclusions for the sponsor.
Conclusions and relevance: This cross-sectional study illustrates how industry involvement in the most influential clinical trials was prominent not only for funding, but also authorship and provision of analysts and was associated with conclusions favoring the sponsor. While most influential trials reported that they planned to share data and make both protocols and statistical analysis plans available, raw data and code were rarely readily available.