Dual-inhibitory domain iCARs improve the efficiency of the AND-NOT gate CAR T strategy
- PMID: 37963244
- PMCID: PMC10666036
- DOI: 10.1073/pnas.2312374120
Dual-inhibitory domain iCARs improve the efficiency of the AND-NOT gate CAR T strategy
Abstract
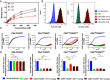
CAR (chimeric antigen receptor) T cell therapy has shown clinical success in treating hematological malignancies, but its treatment of solid tumors has been limited. One major challenge is on-target, off-tumor toxicity, where CAR T cells also damage normal tissues that express the targeted antigen. To reduce this detrimental side-effect, Boolean-logic gates like AND-NOT gates have utilized an inhibitory CAR (iCAR) to specifically curb CAR T cell activity at selected nonmalignant tissue sites. However, the strategy seems inefficient, requiring high levels of iCAR and its target antigen for inhibition. Using a TROP2-targeting iCAR with a single PD1 inhibitory domain to inhibit a CEACAM5-targeting CAR (CEACAR), we observed that the inefficiency was due to a kinetic delay in iCAR inhibition of cytotoxicity. To improve iCAR efficiency, we modified three features of the iCAR-the avidity, the affinity, and the intracellular signaling domains. Increasing the avidity but not the affinity of the iCAR led to significant reductions in the delay. iCARs containing twelve different inhibitory signaling domains were screened for improved inhibition, and three domains (BTLA, LAIR-1, and SIGLEC-9) each suppressed CAR T function but did not enhance inhibitory kinetics. When inhibitory domains of LAIR-1 or SIGLEC-9 were combined with PD-1 into a single dual-inhibitory domain iCAR (DiCARs) and tested with the CEACAR, inhibition efficiency improved as evidenced by a significant reduction in the inhibitory delay. These data indicate that a delicate balance between CAR and iCAR signaling strength and kinetics must be achieved to regulate AND-NOT gate CAR T cell selectivity.
Keywords: AND-NOT logic gate; chimeric antigen receptor; immunotherapy; inhibitory CAR (iCAR); on-target, off-tumor toxicity.
Conflict of interest statement
O.N.W. currently has consulting, equity, and/or board relationships with Trethera Corporation, Kronos Biosciences, Sofie Biosciences, Breakthrough Properties, Vida Ventures, Nammi Therapeutics, Two River, Iconovir, Appia BioSciences, Neogene Therapeutics, 76Bio, and Allogene Therapeutics. T.S. currently has consulting relationships with Dren Bio. A.M.W. currently has consulting, equity, and/or board relationships with ImaginAb and Novartis Institute for Biomedical Research. None of these companies contributed to or directed any of the research reported in this article. A provisional patent listing N.J.B. and O.N.W. has been submitted based on this work through the UCLA Technology Development Group.
Figures




References
-
- Zachery Halford P., A review of CAR T-cell therapies approved for the treatment of relapsed and refractory B-cell lymphomas. J. Hematol. Oncol. Pharm. 13 (2022).
-
- Mullard A., FDA approves second BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discovery 21, 249–249 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

