Modeling the temporal evolution of plasma p-tau in relation to amyloid beta and tau PET
- PMID: 37963289
- PMCID: PMC10916944
- DOI: 10.1002/alz.13539
Modeling the temporal evolution of plasma p-tau in relation to amyloid beta and tau PET
Abstract
Introduction: The timing of plasma biomarker changes is not well understood. The goal of this study was to evaluate the temporal co-evolution of plasma and positron emission tomography (PET) Alzheimer's disease (AD) biomarkers.
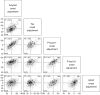
Methods: We included 1408 Mayo Clinic Study of Aging and Alzheimer's Disease Research Center participants. An accelerated failure time (AFT) model was fit with amyloid beta (Aβ) PET, tau PET, plasma p-tau217, p-tau181, and glial fibrillary acidic protein (GFAP) as endpoints.
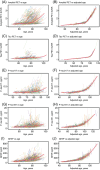
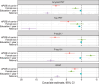
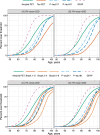
Results: Individual timing of plasma p-tau progression was strongly associated with Aβ PET and GFAP progression. In the population, GFAP became abnormal first, then Aβ PET, plasma p-tau, and tau PET temporal meta-regions of interest when applying cut points based on young, cognitively unimpaired participants.
Discussion: Plasma p-tau is a stronger indicator of a temporally linked response to elevated brain Aβ than of tau pathology. While Aβ deposition and a rise in GFAP are upstream events associated with tau phosphorylation, the temporal link between p-tau and Aβ PET was the strongest.
Highlights: Plasma p-tau progression was more strongly associated with Aβ than tau PET. Progression on plasma p-tau was associated with Aβ PET and GFAP progression. P-tau181 and p-tau217 become abnormal after Aβ PET and before tau PET. GFAP became abnormal first, before plasma p-tau and Aβ PET.
Keywords: Alzheimer's disease; amyloid beta PET; plasma p-tau; tau PET; temporal modeling.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
P.M.C, E.S.L., T.M.T., H.J.W., and J.L.G. have no disclosures. J.G.R. serves on a data safety monitoring board for Strokenet and receives research support from the NIH. A.A.‐S. has participated in advisory boards for Roche Diagnostics, Fujirebio Diagnostics and Siemens Healthineers. C.G.S. receives research support from the NIH. M.L.S. holds stock in medical‐related companies, unrelated to the current work: Align Technology, Inc. He has also owned stock in these medical‐related companies within the past 3 years, unrelated to the current work: LHC Group, Inc. V.J.L. is a consultant for AVID Radiopharmaceuticals, Eisai Co. Inc., Bayer Schering Pharma, GE Healthcare, Piramal Life Sciences, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and NIH (NIA, NCI). M.M.M. has served on scientific advisory boards and/or has consulted for Biogen, LabCorp, Lilly, Merck, Siemens Healthineers, and Sunbird Bio and receives grant support from the NIH and Department of Defense. D.S.K. served on a data safety monitoring board for the DIAN study. He serves on a data safety monitoring board for a tau therapeutic for Biogen but receives no personal compensation. He was a site investigator in the Biogen aducanumab trials. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences but receives no personal compensation. He receives research support from the NIH. P.V. received speaker fees from Miller Medical Communications, Inc. and receives research support from the NIH. R.C.P. serves as a consultant for Roche Inc., Genetech, Inc., Nestle, Inc., Eli Llly and Co., and Eisai, Inc. He serves on the data safety monitoring board for Genentech, Inc. and receives royalties from Oxford University Press, UpToDate, and MedScape. He receives research support from the NIH and the Alzheimer's Association. C.R.J. receives no personal compensation from any commercial entity. He receives research support from the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS097495/NH/NIH HHS/United States
- R01 AG041851/AG/NIA NIH HHS/United States
- RF1 AG069052/AG/NIA NIH HHS/United States
- R01 AG056366/AG/NIA NIH HHS/United States
- R37 AG011378/NH/NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG016574/NH/NIH HHS/United States
- U01 AG006786/NH/NIH HHS/United States
- RF1 AG069052/NH/NIH HHS/United States
- R37 AG011378/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- RO1 AG041851/NH/NIH HHS/United States
- R01 AG056366/NH/NIH HHS/United States
- R01 NS097495/NS/NINDS NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

