Prediction of Adjuvant Gemcitabine Sensitivity in Resectable Pancreatic Adenocarcinoma Using the GemPred RNA Signature: An Ancillary Study of the PRODIGE-24/CCTG PA6 Clinical Trial
- PMID: 37963313
- PMCID: PMC10950182
- DOI: 10.1200/JCO.22.02668
Prediction of Adjuvant Gemcitabine Sensitivity in Resectable Pancreatic Adenocarcinoma Using the GemPred RNA Signature: An Ancillary Study of the PRODIGE-24/CCTG PA6 Clinical Trial
Abstract
Purpose: GemPred, a transcriptomic signature predictive of the efficacy of adjuvant gemcitabine (GEM), was developed from cell lines and organoids and validated retrospectively. The phase III PRODIGE-24/CCTG PA6 trial has demonstrated the superiority of modified folinic acid, fluorouracil, irinotecan, and oxaliplatin (mFOLFIRINOX) over GEM as adjuvant therapy in patients with resected pancreatic ductal adenocarcinoma at the expense of higher toxicity. We evaluated the potential predictive value of GemPred in this population.
Patients and methods: Routine formalin-fixed paraffin-embedded surgical specimens of 350 patients were retrieved for RNA sequencing and GemPred prediction (167 in the GEM arm and 183 in the mFOLFIRINOX [mFFX] arm). Survival analyses were stratified by resection margins, lymph node status, and cancer antigen 19-9 level.
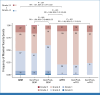
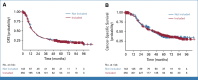
Results: Eighty-nine patients' tumors (25.5%) were GemPred+ and were thus predicted to be gemcitabine-sensitive. In the GEM arm, GemPred+ patients (n = 50, 30%) had a significantly longer disease-free survival (DFS) than GemPred- patients (n = 117, 70%; median 27.3 v 10.2 months, hazard ratio [HR], 0.43 [95% CI, 0.29 to 0.65]; P < .001) and cancer-specific survival (CSS; median 68.4 v 28.6 months, HR, 0.42 [95% CI, 0.27 to 0.66]; P < .001). GemPred had no prognostic value in the mFFX arm. DFS and CSS were similar in GemPred+ patients who received adjuvant GEM and mFFX (median 27.3 v 24.0 months, and 68.4 v 51.4 months, respectively). The statistical interaction between GEM and GemPred+ status was significant for DFS (P = .008) and CSS (P = .004). GemPred+ patients had significantly more adverse events of grade ≥3 in the mFFX arm (76%) compared with those in the GEM arm (40%; P = .001).
Conclusion: This ancillary study of a phase III randomized trial demonstrates that among the quarter of patients with a GemPred-positive transcriptomic signature, survival was comparable with that of mFOLFIRINOX, whereas those receiving adjuvant gemcitabine had fewer adverse events.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


Similar articles
-
Development and validation of AI-assisted transcriptomic signatures to personalize adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma.Ann Oncol. 2024 Sep;35(9):780-791. doi: 10.1016/j.annonc.2024.06.010. Epub 2024 Jun 19. Ann Oncol. 2024. PMID: 38906254
-
A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma.Ann Oncol. 2021 Feb;32(2):250-260. doi: 10.1016/j.annonc.2020.10.601. Epub 2020 Nov 12. Ann Oncol. 2021. PMID: 33188873
-
Preoperative treatment with mFOLFIRINOX or Gemcitabine/Nab-paclitaxel +/- isotoxic high-dose stereotactic body Radiation Therapy (iHD-SBRT) for borderline resectable pancreatic adenocarcinoma (the STEREOPAC trial): study protocol for a randomised comparative multicenter phase II trial.BMC Cancer. 2023 Sep 21;23(1):891. doi: 10.1186/s12885-023-11327-x. BMC Cancer. 2023. PMID: 37735634 Free PMC article.
-
A systematic review and network meta-analysis of phase III randomised controlled trials for adjuvant therapy following resection of pancreatic ductal adenocarcinoma (PDAC).HPB (Oxford). 2020 May;22(5):649-659. doi: 10.1016/j.hpb.2019.12.001. Epub 2019 Dec 29. HPB (Oxford). 2020. PMID: 31894014
-
What else in gemcitabine-pretreated advanced pancreatic cancer? An update of second line therapies.Rev Recent Clin Trials. 2010 Jan;5(1):43-56. doi: 10.2174/157488710790820553. Rev Recent Clin Trials. 2010. PMID: 20205687 Review.
Cited by
-
Total Neoadjuvant Therapy in Localized Pancreatic Cancer: Is More Better?Cancers (Basel). 2024 Jun 30;16(13):2423. doi: 10.3390/cancers16132423. Cancers (Basel). 2024. PMID: 39001485 Free PMC article. Review.
-
Improving patient stratification and selection for curative-intent treatment in localized pancreatic cancer.Hepatobiliary Surg Nutr. 2024 Apr 3;13(2):325-328. doi: 10.21037/hbsn-23-668. Epub 2024 Mar 29. Hepatobiliary Surg Nutr. 2024. PMID: 38617473 Free PMC article. No abstract available.
-
Editorial: Molecular markers for pancreatic cancers: new technologies and applications in the clinical practice.Front Oncol. 2025 Jul 8;15:1651566. doi: 10.3389/fonc.2025.1651566. eCollection 2025. Front Oncol. 2025. PMID: 40697368 Free PMC article. No abstract available.
-
The Clinical Implications of KRAS Mutations and Variant Allele Frequencies in Pancreatic Ductal Adenocarcinoma.J Clin Med. 2024 Apr 4;13(7):2103. doi: 10.3390/jcm13072103. J Clin Med. 2024. PMID: 38610868 Free PMC article. Review.
-
Prognosis Associated with Complete Pathological Response Following Neoadjuvant Treatment for PancreaTic AdenOcarciNOma in the FOFLIRINOX Era: the Multicenter TONO Study.Ann Surg Oncol. 2025 Apr;32(4):2809-2818. doi: 10.1245/s10434-024-16735-2. Epub 2025 Jan 8. Ann Surg Oncol. 2025. PMID: 39777595
References
-
- Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA A Cancer J Clin 72:7-33, 2022 - PubMed
-
- Neoptolemos JP, Stocken DD, Bassi C, et al. : Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 304:1073, 2010 - PubMed
-
- Neoptolemos J, Dunn J, Stocken D, et al. : Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 358:1576-1585, 2001 - PubMed
-
- Oettle H, Post S, Neuhaus P, et al. : Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 297:267, 2007 - PubMed
-
- Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

