Mechanistic insights into glycoside 3-oxidases involved in C-glycoside metabolism in soil microorganisms
- PMID: 37963862
- PMCID: PMC10646112
- DOI: 10.1038/s41467-023-42000-3
Mechanistic insights into glycoside 3-oxidases involved in C-glycoside metabolism in soil microorganisms
Abstract
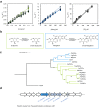
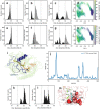
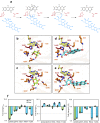
C-glycosides are natural products with important biological activities but are recalcitrant to degradation. Glycoside 3-oxidases (G3Oxs) are recently identified bacterial flavo-oxidases from the glucose-methanol-coline (GMC) superfamily that catalyze the oxidation of C-glycosides with the concomitant reduction of O2 to H2O2. This oxidation is followed by C-C acid/base-assisted bond cleavage in two-step C-deglycosylation pathways. Soil and gut microorganisms have different oxidative enzymes, but the details of their catalytic mechanisms are largely unknown. Here, we report that PsG3Ox oxidizes at 50,000-fold higher specificity (kcat/Km) the glucose moiety of mangiferin to 3-keto-mangiferin than free D-glucose to 2-keto-glucose. Analysis of PsG3Ox X-ray crystal structures and PsG3Ox in complex with glucose and mangiferin, combined with mutagenesis and molecular dynamics simulations, reveal distinctive features in the topology surrounding the active site that favor catalytically competent conformational states suitable for recognition, stabilization, and oxidation of the glucose moiety of mangiferin. Furthermore, their distinction to pyranose 2-oxidases (P2Oxs) involved in wood decay and recycling is discussed from an evolutionary, structural, and functional viewpoint.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

