Bioactivity properties of hydroxyapatite/clay nanocomposites
- PMID: 37963905
- PMCID: PMC10645845
- DOI: 10.1038/s41598-023-45646-7
Bioactivity properties of hydroxyapatite/clay nanocomposites
Abstract
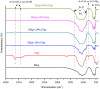
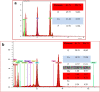
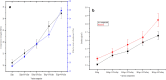
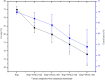
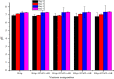
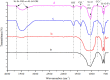
The need for bioactive and non-toxic biomaterials is on a high demand in tissue engineering applications nowadays. Hydroxyapatite (HAp) is the chief constituent of teeth and bones in mammas. One of the major challenges with the use of HAp in engineering application is its brittleness and to overcome this, it's important to react it with a material that can enhanced it's fragility. To this end, HAp and HAp/clay nanocomposites were developed via wet chemical process to mimic natural HAp and to equally confer special properties such as mechanical properties, high surface area, crystallinity, high porosity, and biocompatibility on the biomaterial. The functional groups properties of the as-prepared nanocomposites analyzed by FT-IR showed that the HAp and clay posed reactive centers such as Al-Al-OH, Si-Si-OH, Si-O, PO43-, -OH, and Si-O-Al. The XRD results confirmed the formation of HAp/clay nanocomposite, while SEM and TEM images showed the morphologies of the prepared nanocomposites to be round shape particles. Besides, EDX result revealed the Ca/P ratio of HAp and HAp-C to be lower than that of stoichiometric ratio (1.67) which implies the presence of K, Na, Ca, Mg, Si and Al in the HAp/clay nanocomposite. The mechanical properties of the apatite were greatly enhanced by the addition of clay. The physiological behaviour of the fabricated apatite composites in saline solution showed steady increase in the values of the saline pH of the various biomolecules until day 5 and became fairly constant at day 7 with pH range of 7.30-7.38. Though the saline solution was acidic at the beginning due to dissolved carbon dioxide, the pH of the saline solution containing the nanocomposites gradually became neutral and fairly alkaline over time as a result of the presence of Lewis basis structures in the composites which helps in neutralizing the acidic solution. Furthermore, proliferation of apatites particles onto the surface of the nanocomposites was observed after treatment with simulated body fluids (SBF) media for 7 days. Thus, HAp/clay nanocomposites can be useful biomaterials in bone tissue engineering.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Zhen G, Renfeng W, Zhaoyang L, Zhenduo C, Shengli Z, Yanqin L, Yunde L, Bao H, Xue L, Qianyu H, Zhili L, Xianjin Y. Synthesis, characterization and biological evaluation of strontium/magnesium-co-substituted hydroxyapatite. J. Biomater. Appl. 2016;31:140–151. doi: 10.1177/0885328216633892. - DOI - PubMed
-
- Gražyna SM, Lenka P, Daniela P. Preparation and mechanical properties of polymeric nanocomposites with hydroxyapatite and hydroxyapatite/clay mineral fillers – Review. J. Nanotech. Nanomed. Nanobiotechnol. 2019 doi: 10.24966/NTMB-2044/100007. - DOI
-
- Ofudje EA, Akinwunmi F, Sodiya EF, Alayande SO, Ogundiran AA, Ajayi GO. Biogenic preparation of biphasic calcium phosphate powder from natural source of snail shells: Bioactivity study. SN Appl. Sci. 2022;4:144. doi: 10.1007/s42452-022-05025-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

