Pathogenicity and virulence of human respiratory syncytial virus: Multifunctional nonstructural proteins NS1 and NS2
- PMID: 37964591
- PMCID: PMC12506921
- DOI: 10.1080/21505594.2023.2283897
Pathogenicity and virulence of human respiratory syncytial virus: Multifunctional nonstructural proteins NS1 and NS2
Abstract
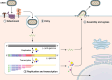
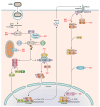
Human respiratory syncytial virus (hRSV) is a major cause of lower respiratory tract infections in young children, the elderly, and immunocompromised. Despite its discovery over 60 years ago and the global impact on human health, limited effective prophylactic or therapeutic options have been available for hRSV infections. This has largely been attributed to the legacy of vaccine failure in the 1960s using a formalin-inactivated RSV , which led to enhancement of disease post exposure to hRSV infection and hampered subsequent development of vaccine candidates. Recent FDA approval of vaccines for older adults and pregnant individuals are major advancements but leaves children between 6 months and 5 years of age unprotected. Part of this limitation is due to a lack of complete understanding of the factors that contribute to hRSV pathogenesis. The nonstructural proteins NS1 and NS2 are multifunctional virulence factors unique to hRSV and that play critical roles during hRSV infection, including antagonizing interferon (IFN) signaling to modulate host responses to hRSV infection. However, the molecular mechanisms by which the nonstructural proteins mediate their IFN inhibitory functions have not been completely defined. Current progress on the characterization of NS1 and NS2 during infection provides deeper insight into their roles. Furthermore, reverse genetics systems for hRSV provide a viable strategy to generate attenuated viruses by introduction of select mutations while maintaining immunogenicity required to elicit a long-term protective response. Here, we will review the current state of knowledge of the nonstructural proteins, their contributions to RSV pathogenesis, and their potential as targets for therapeutic development.
Keywords: Human respiratory syncytial virus; NS1; NS2; nonstructural proteins; viral IFN antagonists.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
