The development of a nucleus pulposus-derived cartilage analog scaffold for chondral repair and regeneration
- PMID: 37964720
- PMCID: PMC10842041
- DOI: 10.1002/jbm.a.37639
The development of a nucleus pulposus-derived cartilage analog scaffold for chondral repair and regeneration
Abstract
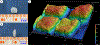
Focal chondral defects (FCDs) significantly impede quality of life for patients and impose severe economic costs on society. One of the most promising treatment options-autologous matrix-induced chondrogenesis (AMIC)-could benefit from a scaffold that contains both of the primary cartilage matrix components-sulfated glycosaminoglycans (sGAGs) and collagen type II. Here, 17 different protocols were evaluated to determine the most optimum strategy for decellularizing (decelling) the bovine nucleus pulposus (bNP) to yield a natural biomaterial with a cartilaginous constituency. The resulting scaffold was then characterized with respect to its biochemistry, biomechanics and cytocompatibility. Results indicated that the optimal decell protocol involved pre-crosslinking the tissue prior to undergoing decell with trypsin and Triton X-100. The residual DNA content of the scaffold was found to be 32.64 ± 9.26 ng/mg dry wt. of tissue with sGAG and hydroxyproline (HYP) contents of 72.53 ± 16.43. and 78.38 ± 8.46 μg/mg dry wt. respectively. The dynamic viscoelastic properties were found to be preserved (complex modulus: 17.92-16.62 kPa across a range of frequencies) while the equilibrium properties were found to have significantly decreased (aggregate modulus: 11.51 ± 9.19 kPa) compared to the non-decelled fresh bNP tissue. Furthermore, the construct was also found to be cytocompatible with bone marrow stem cells (BMSCs). While it was not permissive of cellular infiltration, the BMSCs were still found to have lined the laser drilled channels in the scaffold. Taken together, the biomaterial developed herein could be a valuable addition to the AMIC family of scaffolds or serve as an off-the-shelf standalone option for cartilage repair.
Keywords: autologous matrix-induced chondrogenesis; bovine nucleus pulposus; cartilage; decellularization; focal chondral defect.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflict of interest.
Figures



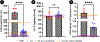



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

