Engineering a dual vaccine against COVID-19 and tuberculosis
- PMID: 37965265
- PMCID: PMC10641007
- DOI: 10.3389/fcimb.2023.1273019
Engineering a dual vaccine against COVID-19 and tuberculosis
Abstract
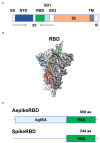
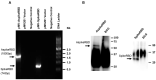
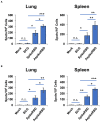
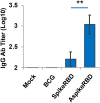
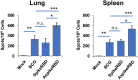
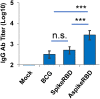
The COVID-19 pandemic, caused by SARS-CoV-2 virus, has been one of the top public health threats across the world over the past three years. Mycobacterium bovis BCG is currently the only licensed vaccine for tuberculosis, one of the deadliest infectious diseases in the world, that is caused by Mycobacterium tuberculosis. In the past decades, recombinant M.bovis BCG has been studied as a novel vaccine vector for other infectious diseases in humans besides tuberculosis, such as viral infections. In the current study, we generated a recombinant M. bovis BCG strain AspikeRBD that expresses a fusion protein consisting of M. tb Ag85A protein and the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein using synthetic biology technique. Our results show that the recombinant M. bovis BCG strain successfully expressed this fusion protein. Interestingly, the recombinant M. bovis BCG strain AspikeRBD significantly induced SARS-CoV-2 spike-specific T cell activation and IgG production in mice when compared to the parental M.bovis BCG strain, and was more potent than the recombinant M.bovis BCG strain expressing SARS-CoV-2 spike RBD alone. As expected, the recombinant M. bovis BCG strain AspikeRBD activated an increased number of M. tb Ag85A-specific IFNγ-releasing T cells and enhanced IgG production in mice when compared to the parental M.bovis BCG strain or the BCG strain expressing SARS-CoV-2 spike RBD alone. Taken together, our results indicate a potential application of the recombinant M. bovis BCG strain AspikeRBD as a novel dual vaccine against SARS-CoV-2 and M. tb in humans.
Keywords: COVID-19; Mycobacterium bovis BCG; dual vaccine; mice; tuberculosis.
Copyright © 2023 Guthrie, Tan, Meeker, Self, Liu and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arts R. J. W., Moorlag S., Novakovic B., Li Y., Wang S. Y., Oosting M., et al. . (2018). BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 23 (1), 89–100.e105. doi: 10.1016/j.chom.2017.12.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

