Cytotoxic CD4+ T cells in chronic viral infections and cancer
- PMID: 37965314
- PMCID: PMC10642198
- DOI: 10.3389/fimmu.2023.1271236
Cytotoxic CD4+ T cells in chronic viral infections and cancer
Abstract
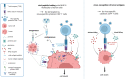
CD4+ T cells play an important role in immune responses against pathogens and cancer cells. Although their main task is to provide help to other effector immune cells, a growing number of infections and cancer entities have been described in which CD4+ T cells exhibit direct effector functions against infected or transformed cells. The most important cell type in this context are cytotoxic CD4+ T cells (CD4+ CTL). In infectious diseases anti-viral CD4+ CTL are mainly found in chronic viral infections. Here, they often compensate for incomplete or exhausted CD8+ CTL responses. The induction of CD4+ CTL is counter-regulated by Tregs, most likely because they can be dangerous inducers of immunopathology. In viral infections, CD4+ CTL often kill via the Fas/FasL pathway, but they can also facilitate the exocytosis pathway of killing. Thus, they are very important effectors to keep persistent virus in check and guarantee host survival. In contrast to viral infections CD4+ CTL attracted attention as direct anti-tumor effectors in solid cancers only recently. Anti-tumor CD4+ CTL are defined by the expression of cytolytic markers and have been detected within the lymphocyte infiltrates of different human cancers. They kill tumor cells in an antigen-specific MHC class II-restricted manner not only by cytolysis but also by release of IFNγ. Thus, CD4+ CTL are interesting tools for cure approaches in chronic viral infections and cancer, but their potential to induce immunopathology has to be carefully taken into consideration.
Keywords: CD4 CTL; CD4 T cells; cancer; chronic viral infections; cytotoxicity.
Copyright © 2023 Malyshkina, Brüggemann, Paschen and Dittmer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

