Vaccine-induced SARS-CoV-2 antibody response: the comparability of S1-specific binding assays depends on epitope and isotype discrimination
- PMID: 37965324
- PMCID: PMC10641008
- DOI: 10.3389/fimmu.2023.1257265
Vaccine-induced SARS-CoV-2 antibody response: the comparability of S1-specific binding assays depends on epitope and isotype discrimination
Abstract
Background: Quantification of the SARS-CoV-2-specific immune response by serological immunoassays is critical for the management of the COVID-19 pandemic. In particular, neutralizing antibody titers to the viral spike (S) protein have been proposed as a correlate of protection (CoP). The WHO established the First International Standard (WHO IS) for anti-SARS-CoV-2 immunoglobulin (Ig) (NIBSC 20/136) to harmonize binding assays with the same antigen specificity by assigning the same unitage in binding antibody units (BAU)/ml.
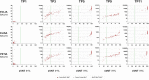
Method: In this study, we analyzed the S1-specific antibody response in a cohort of healthcare workers in Germany (n = 76) during a three-dose vaccination course over 8.5 months. Subjects received either heterologous or homologous prime-boost vaccination with ChAdOx1 nCoV-19 (AstraZeneca) and BNT162b2 (Pfizer-BioNTech) or three doses of BNT162b2. Antibodies were quantified using three anti-S1 binding assays (ELISA, ECLIA, and PETIA) harmonized to the WHO IS. Serum levels of neutralizing antibodies were determined using a surrogate virus neutralization test (sVNT). Binding assays were compared using Spearman's rank correlation and Passing-Bablok regression.
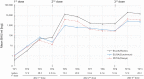
Findings: All assays showed good correlation and similar antibody kinetics correlating with neutralizing potential. However, the assays show large proportional differences in BAU/ml. ECLIA and PETIA, which detect total antibodies against the receptor- binding domain (RBD) within the S1 subunit, interact similarly with the convalescent plasma-derived WHO IS but differently with vaccine serum, indicating a high sensitivity to the IgG/IgM/IgA ratio.
Conclusion: All three binding assays allow monitoring of the antibody response in COVID-19-vaccinated individuals. However, the assay-specific differences hinder the definition of a common protective threshold in BAU/ml. Our results highlight the need for the thoughtful use of conversion factors and consideration of method-specific differences. To improve the management of future pandemics and harmonize total antibody assays, we should strive for reference material with a well-characterized Ig isotype composition.
Keywords: COVID-19 vaccines; SARS-CoV-2 antibody; WHO standard; correlate of protection; humoral immune response; neutralizing antibodies; serological testing; spike protein.
Copyright © 2023 Schest, Langer, Stiegler, Karnuth, Arends, Stiegler, Masetto, Peter and Grimmler.
Conflict of interest statement
Authors SS, CL, YS, BK, JA, and HS are employed at Medizinisches Versorgungszentrum für Labormedizin und Mikrobiologie Ruhr GmbH Essen, Germany. MG is employed at DiaServe Laboratories GmbH Iffeldorf, Germany. MG and TM are employees of DiaSys Diagnostic GmbH Holzheim, Germany and are named as inventors on a patent application Deutsche Patentanmeldung 10 2020 122 593.8 claiming the manufacturing and use of the described PETIA for serological quantification of anti-SARS-CoV-2 antibodies. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study.Lancet Respir Med. 2021 Nov;9(11):1255-1265. doi: 10.1016/S2213-2600(21)00357-X. Epub 2021 Aug 13. Lancet Respir Med. 2021. PMID: 34391547 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
Serological Correlates of Protection Induced by COVID-19 Vaccination in the Working Age Population: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2024 May 3;12(5):494. doi: 10.3390/vaccines12050494. Vaccines (Basel). 2024. PMID: 38793745 Free PMC article. Review.
-
Aptamers as promising nanotheranostic tools in the COVID-19 pandemic era.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 May;14(3):e1785. doi: 10.1002/wnan.1785. Epub 2022 Mar 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 35238490 Free PMC article. Review.
References
-
- World Health Organization . WHO COVID-19 Dashboard. Available at: https://covid19.who.int/ (Accessed June 27, 2023).
-
- World Health Organization . COVID-19 Vaccines with WHO Emergency Use Listing. Available at: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (Accessed June 27, 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

