Prevention of CMV/EBV reactivation by double-specific T cells in patients after allogeneic stem cell transplantation: results from the randomized phase I/IIa MULTIVIR-01 study
- PMID: 37965339
- PMCID: PMC10642256
- DOI: 10.3389/fimmu.2023.1251593
Prevention of CMV/EBV reactivation by double-specific T cells in patients after allogeneic stem cell transplantation: results from the randomized phase I/IIa MULTIVIR-01 study
Abstract
Introduction: Allogeneic stem cell transplantation is used to cure hematologic malignancies or deficiencies of the hematopoietic system. It is associated with severe immunodeficiency of the host early after transplant and therefore early reactivation of latent herpesviruses such as CMV and EBV within the first 100 days are frequent. Small studies and case series indicated that application of herpes virus specific T cells can control and prevent disease in this patient population.
Methods: We report the results of a randomized controlled multi centre phase I/IIa study (MULTIVIR-01) using a newly developed T cell product with specificity for CMV and EBV derived from the allogeneic stem cell grafts used for transplantation. The study aimed at prevention and preemptive treatment of both viruses in patients after allogeneic stem cell transplantation targeting first infusion on day +30. Primary endpoints were acute transfusion reaction and acute-graft versus-host-disease after infusion of activated T cells.
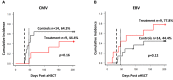
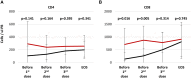
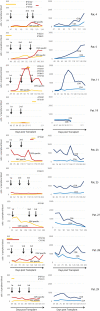
Results: Thirty-three patients were screened and 9 patients were treated with a total of 25 doses of the T cell product. We show that central manufacturing can be achieved successfully under study conditions and the product can be applied without major side effects. Overall survival, transplant related mortality, cumulative incidence of graft versus host disease and number of severe adverse events were not different between treatment and control groups. Expansion of CMV/EBV specific T cells was observed in a fraction of patients, but overall there was no difference in virus reactivation.
Discussion: Our study results indicate peptide stimulated epitope specific T cells derived from stem cell grafts can be administered safely for prevention and preemptive treatment of reactivation without evidence for induction of acute graft versus host disease.
Clinical trial registration: https://clinicaltrials.gov, identifier NCT02227641.
Keywords: Epstein-Barr virus EBV; allogeneic; cytomegalovirus CMV; epitope specificity; prevention; reactivation; stem cell transplantation (SCT).
Copyright © 2023 Gerbitz, Gary, Aigner, Moosmann, Kremer, Schmid, Hirschbuehl, Wagner, Hauptrock, Teschner, Roesler, Spriewald, Tischer, Moi, Balzer, Schaffer, Bausenwein, Wagner, Schmidt, Brestrich, Ullrich, Maas, Herold, Strobel, Zimmermann, Weisbach, Hansmann, Lammoglia-Cobo, Remberger, Stelljes, Ayuk, Zeiser and Mackensen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures