Modulating asthma-COPD overlap responses with IL-17 inhibition
- PMID: 37965351
- PMCID: PMC10641519
- DOI: 10.3389/fimmu.2023.1271342
Modulating asthma-COPD overlap responses with IL-17 inhibition
Abstract
Background: IL-17 is a modulator of the inflammatory response and is implicated in lung remodeling in both asthma and chronic obstructive pulmonary disease (COPD). Well as and probably in patients with asthma-COPD overlap (ACO).
Methods: In this study, we evaluated the response of the airways and alveolar septa to anti-IL-17 treatment in an ACO model. Fifty-six male BALB/c mice were sensitized with ovalbumin (OVA group), received porcine pancreatic elastase (PPE group), or both (ACO group). Mice were then treated with either anti-IL-17 monoclonal antibody or saline. We evaluated hyperresponsiveness, bronchoalveolar lavage fluid (BALF) cell counts, and mean alveolar diameter. We quantified inflammatory, response, extracellular matrix remodeling, oxidative stress markers, and signaling pathway markers.
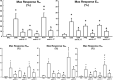
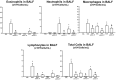
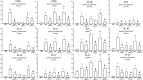
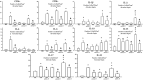
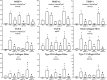
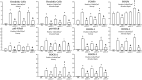
Results: Anti-IL-17 treatment in the ACO anti-IL-17 group reduced the maximum response of respiratory system Rrs, Ers, Raw, Gtis, this when compared to the ACO group (p<0.05). There was a reduction in the total number of inflammatory cells, neutrophils, and macrophages in the BALF in the ACO anti-IL-17 group compared to the ACO group (p<0.05). There was attenuated dendritic cells, CD4+, CD8+, FOXP3, IL-1β, IL-2, IL-6, IL-13, IL-17, IL-33 in ACO anti-IL-17 group in airway and alveolar septum compared to the ACO group (p<0.05). We observed a reduction of MMP-9, MMP-12, TIMP-1, TGF-β, collagen type I in ACO anti-IL-17 group in airway and alveolar septum compared to the ACO group (p < 0.05). We also observed a reduction of iNOS and 8-iso-PGF2α in the airways and in the alveolar septum was reduced in the ACO anti-IL-17group compared to the ACO group (p < 0.05). Regarding the signaling pathways, NF-kB, ROCK-1, and ROCK-2 in the airway and alveolar septum were attenuated in the ACO anti-IL-17 group when compared to the ACO group (p<0.05).
Conclusions: Our results suggest that inhibiting IL-17 modulates cell-associated cytokine production in lung tissue, extracellular matrix remodeling, and oxidative stress in ACO through the modulation of NF-kB and FOXP3.
Keywords: anti-IL-17; asthma; asthma-COPD overlap (ACO); extracellular matrix remodeling; inflammation; oxidative stress.
Copyright © 2023 Camargo, Righetti, de Almeida, Santos, Fukuzaki, Martins, Barbeiro, Saraiva-Romanholo, Lopes, Leick, Prado and Tibério.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- GINA . The global strategy for asthma management and prevention. Global Initiative Asthma (2021).
-
- Montes de Oca ,M, Lopez Varela MV, Menezes AMB, Wehrmeister FC, Ramirez L, Miravitlles M. Respiratory symptoms (COPD Assessment Test and modified Medical Research Council dyspnea scores) and GOLD-ABCD COPD classification: the LASSYC study. Jornal Brasileiro Pneumol (2021), e20210156. doi: 10.36416/1806-3756/e20210156 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

