Ensuring the quality of medicines in India: An update on the development, modernization, and harmonization of drug standards in the Indian Pharmacopoeia
- PMID: 37965488
- PMCID: PMC10641554
- DOI: 10.1016/j.jsps.2023.101825
Ensuring the quality of medicines in India: An update on the development, modernization, and harmonization of drug standards in the Indian Pharmacopoeia
Abstract
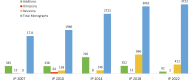
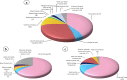
India has a sparkling pharmaceutical sector that holds a distinguished place by producing and supplying high-quality and affordable medicines across the globe. Ensuring the quality and safety of the marketed medicinal products is one of the most important components of the drug regulatory framework and assessment of the quality of medicines is usually achieved by referring to the public standards of the official Pharmacopoeia. In India, the Indian Pharmacopoeia (IP) is published at regular intervals to fulfill the requirements of the Drugs and Cosmetics Act, 1940 to ensure the quality of medicines being manufactured and/or marketed in India. The present article aims to provide an overview of the history of the IP, its standards-setting process, and the current status of monographs in the 9th edition of the IP 2022. Special focus is placed on the newly added and upgraded general chapters and monographs within the IP 2022. There are a total of 223 general chapters and 3152 drug monographs available under various categories in the IP 2022. This study also highlights a total of 92 new drug monograph additions and 412 monograph revisions in the IP 2022. It is anticipated that the standards laid down in the IP 2022 will play an imperative role in delivering quality medicines to patients within and outside India.
Keywords: Active pharmaceutical ingredients; Finished pharmaceutical preparations; Indian pharmacopoeia; Monograph; Reference standards.
© 2023 Published by Elsevier B.V. on behalf of King Saud University.
Conflict of interest statement
The authors declare no conflict of interest. The views expressed in this article are the personal views of the authors. They shall not be understood or quoted as being made on behalf of or reflecting the position of the Indian Pharmacopoeia.
Figures
Similar articles
-
Catering the Need of Drug Manufacturing Standard in India: An Update to Indian Pharmacopoeia.Ther Innov Regul Sci. 2025 May;59(3):471-488. doi: 10.1007/s43441-025-00759-1. Epub 2025 Feb 21. Ther Innov Regul Sci. 2025. PMID: 39984761 Review.
-
Quality Standards and Current Status of Ophthalmic Formulations in Indian Pharmacopoeia and National Formulary of India.Ther Innov Regul Sci. 2014 May;48(3):386-392. doi: 10.1177/2168479013513455. Ther Innov Regul Sci. 2014. PMID: 30235542
-
A Comparative Study of Pharmacopoeial Quality Standards and Regulations of Radiopharmaceuticals.Indian J Nucl Med. 2021 Apr-Jun;36(2):153-162. doi: 10.4103/ijnm.ijnm_222_20. Epub 2021 Jun 21. Indian J Nucl Med. 2021. PMID: 34385786 Free PMC article. Review.
-
Regulatory Requirements for Quality Control of Unani Medicines.J AOAC Int. 2020 Jun 1;103(3):634-648. doi: 10.5740/jaoacint.19-0285. J AOAC Int. 2020. PMID: 31561755
-
[Cannabis for medicinal use: development of pharmacopoeia monographs as a quality standard].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019 Jul;62(7):806-810. doi: 10.1007/s00103-019-02963-5. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019. PMID: 31139840 Review. German.
Cited by
-
Catering the Need of Drug Manufacturing Standard in India: An Update to Indian Pharmacopoeia.Ther Innov Regul Sci. 2025 May;59(3):471-488. doi: 10.1007/s43441-025-00759-1. Epub 2025 Feb 21. Ther Innov Regul Sci. 2025. PMID: 39984761 Review.
References
-
- Anonymous, 2021. Process for Development of Indian Pharmacopoeia Monographs. Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Govt. of India. [Internet] https://ipc.gov.in/images/Process_for_Developmenof_IP_Monograph-GD-02.pdf. Accessed on 15/03/2023.
-
- Anonymous, 2022. Pharma Industry in India: Pharma Sector Overview, Market Size, Analysis. India Brand Equity Foundation (IBEF). [Internet] https://www.ibef.org/industry/pharmaceutical-india. Accessed on 12/04/2023.
-
- Chandler D.K., Volokhov D.V., Chizhikov V.E. Mycoplasma - Historical overview of mycoplasma testing for production of biologics. Am. Pharm. Rev. 2011;14(4):48.
-
- Drugs and Cosmetics Act, 2016. The Drugs and Cosmetics Act and Rules, Ministry of Health & Family welfare, Government of India.
-
- FDA, 2020. FDA updates on hand sanitizers consumers should not use. Centre for Drug Evaluation and Research, U.S. Food and Drug Administration. [Internet] https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-.... Accessed on 15/05/2023.
Publication types
LinkOut - more resources
Full Text Sources