The type-2 Streptococcus canis M protein SCM-2 binds fibrinogen and facilitates antiphagocytic properties
- PMID: 37965557
- PMCID: PMC10641296
- DOI: 10.3389/fmicb.2023.1228472
The type-2 Streptococcus canis M protein SCM-2 binds fibrinogen and facilitates antiphagocytic properties
Abstract
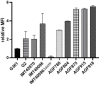
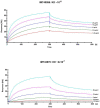
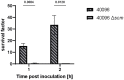
Streptococcus canis is a zoonotic agent that causes severe invasive diseases in domestic animals and humans, but little is known about its pathogenesis and virulence mechanisms so far. SCM, the M-like protein expressed by S. canis, is considered one of the major virulence determinants. Here, we report on the two distinct groups of SCM. SCM-1 proteins were already described to interact with its ligands IgG and plasminogen as well as with itself and confer antiphagocytic capability of SCM-1 expressing bacterial isolates. In contrast, the function of SCM-2 type remained unclear to date. Using whole-genome sequencing and subsequent bioinformatics, FACS analysis, fluorescence microscopy and surface plasmon resonance spectrometry, we demonstrate that, although different in amino acid sequence, a selection of diverse SCM-2-type S. canis isolates, phylogenetically representing the full breadth of SCM-2 sequences, were able to bind fibrinogen. Using targeted mutagenesis of an SCM-2 isolate, we further demonstrated that this strain was significantly less able to survive in canine blood. With respect to similar studies showing a correlation between fibrinogen binding and survival in whole blood, we hypothesize that SCM-2 has an important contribution to the pathogenesis of S. canis in the host.
Keywords: SCM; Streptococcus canis; anti-phagocytosis; fibrinogen-binding; pathogen-host interaction.
Copyright © 2023 Lapschies, Aubry, Kohler, Goldmann, Hammerschmidt, Nerlich, Eichhorn, van Vorst and Fulde.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Andrews S. (2010). “FastQC” in A quality control tool for high throughput sequence data. Cambridge, United Kingdom. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Bergmann R., Jentsch M. C., Uhlig A., Müller U., van der Linden M., Rasmussen M., et al. (2019). Prominent binding of human and equine fibrinogen to Streptococcus equi subsp. zooepidemicus is mediated by specific SzM types and is a distinct phenotype of zoonotic isolates. Infect. Immun. 88:e00559. doi: 10.1128/IAI.00559-19, PMID: - DOI - PMC - PubMed
-
- Brennan-Krohn T. (2021). Infections in animals and humans caused by bacterial 'Cousins'. Washington, DC, United States: American Society for Microbiology.
LinkOut - more resources
Full Text Sources

