Unravelling the functional development of vertebrate pathways controlling gaze
- PMID: 37965576
- PMCID: PMC10640995
- DOI: 10.3389/fcell.2023.1298486
Unravelling the functional development of vertebrate pathways controlling gaze
Abstract
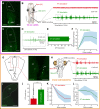
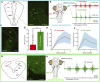
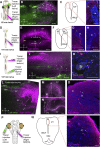
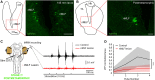
Animals constantly redirect their gaze away or towards relevant targets and, besides these goal-oriented responses, stabilizing movements clamp the visual scene avoiding image blurring. The vestibulo-ocular (VOR) and the optokinetic reflexes are the main contributors to gaze stabilization, whereas the optic tectum integrates multisensory information and generates orienting/evasive gaze movements in all vertebrates. Lampreys show a unique stepwise development of the visual system whose understanding provides important insights into the evolution and development of vertebrate vision. Although the developmental emergence of the visual components, and the retinofugal pathways have been described, the functional development of the visual system and the development of the downstream pathways controlling gaze are still unknown. Here, we show that VOR followed by light-evoked eye movements are the first to appear already in larvae, despite their burrowed lifestyle. However, the circuits controlling goal-oriented responses emerge later, in larvae in non-parasitic lampreys but during late metamorphosis in parasitic lampreys. The appearance of stabilizing responses earlier than goal-oriented in the lamprey development shows a stepwise transition from simpler to more complex visual systems, offering a unique opportunity to isolate the functioning of their underlying circuits.
Keywords: eye movements; goal-oriented movements; lamprey; optic tectum; optokinetic reflex; pretectum; vestibulo-ocular reflex; visual system.
Copyright © 2023 Barandela, Núñez-González, Suzuki, Jiménez-López, Pombal and Pérez-Fernández.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Conserved subcortical processing in visuo-vestibular gaze control.Nat Commun. 2022 Aug 10;13(1):4699. doi: 10.1038/s41467-022-32379-w. Nat Commun. 2022. PMID: 35948549 Free PMC article.
-
The stepwise development of the lamprey visual system and its evolutionary implications.Biol Rev Camb Philos Soc. 2018 Aug;93(3):1461-1477. doi: 10.1111/brv.12403. Epub 2018 Feb 28. Biol Rev Camb Philos Soc. 2018. PMID: 29488315 Review.
-
Functional coupling of the stabilizing gaze reflexes during vertical linear motion in the alert cat.Prog Brain Res. 1989;80:385-94; discussion 373-5. doi: 10.1016/s0079-6123(08)62234-7. Prog Brain Res. 1989. PMID: 2634278
-
Vestibuloocular reflex signal modulation during voluntary and passive head movements.J Neurophysiol. 2002 May;87(5):2337-57. doi: 10.1152/jn.2002.87.5.2337. J Neurophysiol. 2002. PMID: 11976372
-
Role of locomotor efference copy in vertebrate gaze stabilization.Front Neural Circuits. 2022 Dec 9;16:1040070. doi: 10.3389/fncir.2022.1040070. eCollection 2022. Front Neural Circuits. 2022. PMID: 36569798 Free PMC article. Review.
Cited by
-
Direct retino-iridal projections and intrinsic iris contraction mediate the pupillary light reflex in early vertebrates.Commun Biol. 2024 Aug 14;7(1):993. doi: 10.1038/s42003-024-06699-0. Commun Biol. 2024. PMID: 39143195 Free PMC article.
References
-
- Barreiro-Iglesias A., Fernández-López B., Sobrido-Cameán D., Anadón R. (2017). Organization of alpha-transducin immunoreactive system in the brain and retina of larval and young adult Sea Lamprey (Petromyzon marinus), and their relationship with other neural systems. J. Comp. Neurol. 52517, 3683–3704. 10.1002/cne.24296 - DOI - PubMed
-
- Dayton G. O. J., Jones M. H., Aiu P., Rawson R. A., Steele B., Rose M. (1964). Developmental study of coordinated eye movements in the human infant. I. Visual acuity in the newborn human: a study based on induced optokinetic nystagmus recorded by electro-oculography. Arch. Ophthalmol. 71, 865–870. 10.1001/archopht.1964.00970010881017 - DOI - PubMed
LinkOut - more resources
Full Text Sources

