Recombinant zoster vaccine in immunocompetent and immunocompromised adults: A review of clinical studies
- PMID: 37965770
- PMCID: PMC10653762
- DOI: 10.1080/21645515.2023.2278362
Recombinant zoster vaccine in immunocompetent and immunocompromised adults: A review of clinical studies
Abstract
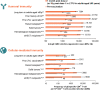
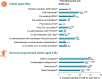
Herpes zoster (HZ) is a debilitating vaccine-preventable disease. Impairment of cell-mediated immunity, as observed with aging and immunosuppressive disorders and therapies, increases risk. Recombinant zoster vaccine (RZV) is efficacious against HZ in adults aged ≥50 years in different settings, and in immunocompromised adults aged ≥18 years who are at increased risk of developing HZ. RZV is the first and only HZ vaccine approved for use in immunocompromised adults globally, including in Europe and the US. RZV has a clinically acceptable safety profile and elicits robust immune responses in adults aged ≥50 years, and in immunocompromised adults aged ≥18 years who are at increased risk of HZ. Additionally, RZV is efficacious against HZ complications such as post-herpetic neuralgia and HZ-related pain. This review updates knowledge from a randomized controlled trial setting on the efficacy, safety, immunogenicity, and impact on quality of life of RZV.
Keywords: Herpes zoster; RZV; immunocompromised; post-herpetic neuralgia; prevention; shingles; vaccine.
Plain language summary
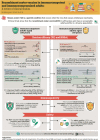
What is the context?The varicella zoster virus, which causes chickenpox in childhood, can reactivate in adults and trigger a painful rash called herpes zoster (HZ) or shingles. Almost all adults are at risk of developing HZ as they age or develop risk factors for HZ. Two key studies published in 2015 and 2016 (ZOE-50 and ZOE-70) compared the recombinant zoster vaccine (RZV) with placebo and showed that RZV could effectively prevent HZ in adults aged ≥50 years and ≥70 years, respectively. Several clinical studies were carried out in subsequent years, assessing how effective and safe RZV is compared with a placebo/control in different populations. Based on these studies, RZV was approved for use in adults aged ≥50 years and those aged ≥18 years at increased risk of HZ (European Union) due to immunodeficiency or immunosuppression caused by known disease or therapy (United States).What is new?We reviewed clinical studies of RZV published between 1 January, 2015 and 31 October, 2022. The evidence shows that RZV is effective and does not cause safety concerns across the studied populations, including adults aged ≥50 years and immunocompromised adults aged ≥18 years who are at increased risk of HZ.What is the impact?The growing amount of knowledge on the efficacy, safety, immunogenicity, and impact on quality of life of RZV should assist in deciding to vaccinate and in ensuring that the individuals who could benefit the most from RZV have access to vaccination.
Conflict of interest statement
All authors are employees of GSK and hold or may hold stock options. NL holds a patent on the herpes zoster vaccine. AS01 is a trademark owned by or licensed to the GSK group of companies.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
