Ventilation tubes (grommets) for otitis media with effusion (OME) in children
- PMID: 37965944
- PMCID: PMC10646987
- DOI: 10.1002/14651858.CD015215.pub2
Ventilation tubes (grommets) for otitis media with effusion (OME) in children
Abstract
Background: Otitis media with effusion (OME) is an accumulation of fluid in the middle ear cavity, common amongst young children. It may cause hearing loss which, when persistent, may lead to developmental delay, social difficulty and poor quality of life. Management includes watchful waiting, autoinflation, medical and surgical treatment. Insertion of ventilation tubes has often been used as the preferred treatment.
Objectives: To evaluate the effects (benefits and harms) of ventilation tubes (grommets) for OME in children.
Search methods: We searched the Cochrane ENT Register, CENTRAL, Ovid MEDLINE, Ovid Embase, Web of Science, ClinicalTrials.gov, ICTRP and additional sources for published and unpublished trials on 20 January 2023.
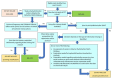
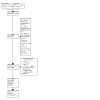
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs in children (6 months to 12 years) with OME for ≥ 3 months. We included studies that compared ventilation tube (VT) insertion with five comparators: no treatment, watchful waiting (ventilation tubes inserted later, if required), myringotomy, hearing aids and other non-surgical treatments.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were determined following a multi-stakeholder prioritisation exercise and were: 1) hearing; 2) OME-specific quality of life; 3) persistent tympanic membrane perforation (as a severe adverse effect of the surgery). Secondary outcomes were: 1) persistence of OME; 2) other adverse effects (including tympanosclerosis, VT blockage and pain); 3) receptive language skills; 4) speech development; 5) cognitive development; 6) psychosocial skills; 7) listening skills; 8) generic health-related quality of life; 9) parental stress; 10) vestibular function; 11) episodes of acute otitis media. We used GRADE to assess the certainty of evidence for key outcomes. Although we included all measures of hearing assessment, the proportion of children who returned to normal hearing was our preferred method, due to challenges in interpreting the results of mean hearing thresholds.
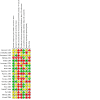
Main results: We included 19 RCTs (2888 children). We considered most of the evidence to be very uncertain, due to wide confidence intervals for the effect estimates, few participants, and a risk of performance and detection bias. Here we report our key outcomes at the longest reported follow-up. There were some limitations to the evidence. No studies investigated the comparison of ventilation tubes versus hearing aids. We did not identify any data on disease-specific quality of life; however, many studies were conducted before the development of specific tools to assess this in otitis media. Short-acting ventilation tubes were used in most studies and thus specific data on the use of long-acting VTs is limited. Finally, we did not identify specific data on the effects of VTs in children at increased risk of OME (e.g. with craniofacial syndromes). Ventilation tubes versus no treatment (four studies) The odds ratio (OR) for a return to normal hearing after 12 months was 1.13 with VTs (95% confidence interval (CI) 0.46 to 2.74; 54% versus 51%; 1 study, 72 participants; very low-certainty evidence). At six months, VTs may lead to a large reduction in persistent OME (risk ratio (RR) 0.30, 95% CI 0.14 to 0.65; 20.4% versus 68.0%; 1 study, 54 participants; low-certainty evidence). The evidence is very uncertain about the chance of persistent tympanic membrane perforation with VTs at 12 months (OR 0.85, 95% CI 0.38 to 1.91; 8.3% versus 9.7%; 1 RCT, 144 participants). Early ventilation tubes versus watchful waiting (six studies) There was little to no difference in the proportion of children whose hearing returned to normal after 8 to 10 years (i.e. by the age of 9 to 13 years) (RR for VTs 0.98, 95% CI 0.94 to 1.03; 93% versus 95%; 1 study, 391 participants; very low-certainty evidence). VTs may also result in little to no difference in the risk of persistent OME after 18 months to 6 years (RR 1.21, 95% CI 0.84 to 1.74; 15% versus 12%; 3 studies, 584 participants; very low-certainty evidence). We were unable to pool data on persistent perforation. One study showed that VTs may increase the risk of perforation after a follow-up duration of 3.75 years (RR 3.65, 95% CI 0.41 to 32.38; 1 study, 391 participants; very low-certainty evidence) but the actual number of children who develop persistent perforation may be low, as demonstrated by another study (1.26%; 1 study, 635 ears; very low-certainty evidence). Ventilation tubes versus non-surgical treatment (one study) One study compared VTs to six months of antibiotics (sulphisoxazole). No data were available on return to normal hearing, but final hearing thresholds were reported. At four months, the mean difference was -5.98 dB HL lower (better) for those receiving VTs, but the evidence is very uncertain (95% CI -9.21 to -2.75; 1 study, 125 participants; very low-certainty evidence). No evidence was identified regarding persistent OME. VTs may result in a low risk of persistent perforation at 18 months of follow-up (no events reported; narrative synthesis of 1 study, 60 participants; low-certainty evidence). Ventilation tubes versus myringotomy (nine studies) We are uncertain whether VTs may slightly increase the likelihood of returning to normal hearing at 6 to 12 months, since the confidence intervals were wide and included the possibility of no effect (RR 1.22, 95% CI 0.59 to 2.53; 74% versus 64%; 2 studies, 132 participants; very low-certainty evidence). After six months, persistent OME may be reduced for those who receive VTs compared to laser myringotomy, but the evidence is very uncertain (OR 0.27, 95% CI 0.19 to 0.38; 1 study, 272 participants; very low-certainty evidence). At six months, the risk of persistent perforation is probably similar with the use of VTs or laser myringotomy (narrative synthesis of 6 studies, 581 participants; moderate-certainty evidence).
Authors' conclusions: There may be small short- and medium-term improvements in hearing and persistence of OME with VTs, but it is unclear whether these persist after longer follow-up. The RCTs included do not allow us to say when (or how much) VTs improve hearing in any specific child. However, interpretation of the evidence is difficult: many children in the control groups recover spontaneously or receive VTs during follow-up, VTs may block or extrude, and OME may recur. The limited evidence in this review also affects the generalisability/applicability of our findings to situations involving children with underlying conditions (e.g. craniofacial syndromes) or the use of long-acting tubes. Consequently, RCTs may not be the best way to determine whether an intervention is likely to be effective in any individual child. Instead, we must better understand the different OME phenotypes to target interventions to children who will benefit most, and avoid over-treating when spontaneous resolution is likely.
Trial registration: ClinicalTrials.gov NCT00365092 NCT02490332.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Samuel MacKeith: ENT private practice (employment); sees patients with general ENT problems in NHS and private practice; Assistant Co‐ordinating Editor of Cochrane ENT 2020‐2023, but had no role in the editorial process for this review.
Caroline A Mulvaney: none known.
Kevin Galbraith: none known.
Katie Webster: none known.
Rachel Connolly: National Institute for Health and Care Excellence (employment: systematic reviewer on the upcoming NICE guideline on otitis media with effusion in under 12s).
Aye Paing: none known.
Tal Marom: no relevant interests; Attending Otolaryngologist.
Mat Daniel: Aventa Med (stock; consultant); Nottingham University Hospitals NHS Trust (employment: ENT consultant); published research papers relevant to the interventions in the work; co‐author of the TARGET trial.
Roderick P Venekamp: no relevant interests; work as a GP; editorial board member of Cochrane ARI and ENT, but had no role in the editorial process for this review.
Maroeska M Rovers: no relevant interests; involved in the KNOOP‐3 study on the effectiveness of ventilation tubes performed in the Netherlands (sponsored by ZonMw).
Anne GM Schilder: joint Co‐ordinating Editor of Cochrane ENT until April 2020, but had no role in the editorial process for this review.; treats patients with OME in her NHS practice. Her evidENT team at the UCL Ear Institute is supported by the National Institute of Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC), with research projects being supported by the NIHR, Wellcome Trust, RNiD, ENT UK and industry; National Specialty Lead for the NIHR Clinical Research Network ENT and Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Research Initiative; in her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she advises CRO, biotech and pharma companies in the hearing field on clinical trial design and delivery.
Figures











































































































































Update of
- doi: 10.1002/14651858.CD015215
References
References to studies included in this review
Bernard 1991 {published data only}
-
- Bernard PA, Stenstrom RJ, Feldman W, Durieux-Smith A. Randomized, controlled trial comparing long-term sulfonamide therapy to ventilation tubes for otitis media with effusion. Pediatrics 1991;88(2):215-22. [CENTRAL: CN-00077175] [PMID: ] - PubMed
-
- Feldman W, Bernard P, Smith A, Stenstrom R. Sulfonamide prophylaxis vs ventilation tubes in hearing-loss due to recurrent otitis-media with effusion (Rome) - preliminary-results of a randomized controlled trial. Clinical and Investigative Medicine-medecine Clinique et Experimentale 1987;4:A33. [CENTRAL: CN-02494379]
-
- Feldman W, Bernard P, Smith A, Stenstrom R. Sulfonamide prophylaxis vs ventilation tubes in hearing-loss due to recurrent otitis-media with effusion - preliminary-results of a randomized controlled trial. American Journal of Diseases of Children 1987;4:389-9. [CENTRAL: CN-02494378]
D'Eredita 2006 {published data only}
Dempster 1993 {published data only}
-
- Dempster JH, Browning GG, Gatehouse SG. A randomized study of the surgical management of children with persistent otitis media with effusion associated with a hearing impairment. Journal of Laryngology and Otology 1993;107(4):284-9. [CENTRAL: CN-00094188] [PMID: ] - PubMed
Elkholy 2021 {published data only}
-
- Elkholy TA, El-gaber A, Mohammed F, Al-Agamy DMM, Elhady M. Impact of adenoidectomy alone or with ventilation tube for treatment of secretory otitis media in children. Al-azhar International Medical Journal 2021;2(8):53-8. [CENTRAL: CN-02518323]
Gates 1989 {published data only}
-
- Gates GA, Avery CA, Cooper JC, Prihoda TJ, Cooper JCJ. Chronic secretory otitis media: effects of surgical management. Annals of Otology, Rhinology & Laryngology. Supplement 1989;138:2-32. [CENTRAL: CN-00057471] [PMID: ] - PubMed
-
- Gates GA, Avery CA, Prihoda TJ, Cooper JC. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. New England Journal of Medicine 1987;317(23):1444-51. [CENTRAL: CN-00051110] [PMID: ] - PubMed
-
- Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope 1988;98(1):58-63. [CENTRAL: CN-00051547] [PMID: ] - PubMed
-
- Gates GA, Wachtendorf C, Hearne EM, Holt GR. Treatment of chronic otitis media with effusion: results of tympanostomy tubes. American Journal of Otolaryngology 1985;6(3):249-53. [CENTRAL: CN-00038728] [PMID: ] - PubMed
Koopman 2004 {published data only}
-
- Koopman JP, Blom H, Hoeve H, Mulder P. Laser myringotomy versus ventilation tubes in children with chronic otitis media with effusion: a randomized trial for efficacy and outcome prediction. In: 8th International Symposium on Recent Advances in Otitis Media. 2003:136. [CENTRAL: CN-00449322]
-
- Koopman JP, Reuchlin AG, Kummer EE, Boumans LJ, Rijntjes E, Hoeve LJ, et al. Laser myringotomy versus ventilation tubes in children with otitis media with effusion: a randomized trial. Laryngoscope 2004;114(5):844-9. [CENTRAL: CN-00469076] [PMID: ] - PubMed
Maw 1983 {published data only}
-
- Maw AR, Herod F. Otoscopic, impedance, and audiometric findings in glue ear treated by adenoidectomy and tonsillectomy. A prospective randomised study. Lancet 1986;1(8495):1399-402. [CENTRAL: CN-00043077] [PMID: ] - PubMed
-
- Maw AR, Parker A. Surgery of the tonsils and adenoids in relation to secretory otitis media in children. Acta Oto-laryngologica. Supplementum 1988;454:202-7. [CENTRAL: CN-00057952] [PMID: ] - PubMed
-
- Maw AR. Age and adenoid size in relation to adenoidectomy in otitis media with effusion. American Journal of Otolaryngology 1985;6(3):245-8. [CENTRAL: CN-00038727] [PMID: ] - PubMed
-
- Maw AR. Chronic otitis media with effusion and adeno-tonsillectomy--a prospective randomized controlled study. International Journal of Pediatric Otorhinolaryngology 1983;6(3):239-46. [CENTRAL: CN-00034535] [PMID: ] - PubMed
Maw 1999 {published data only}
-
- Hall AJ, Maw AR, Steer CD. Developmental outcomes in early compared with delayed surgery for glue ear up to age 7 years: a randomised controlled trial. Clinical Otolaryngology 2009;34(1):12-20. [CENTRAL: CN-00699827] [PMID: ] - PubMed
-
- Maw R, Wilks J, Harvey I, Peters TJ, Golding J, Wiks J. Early surgery compared with watchful waiting for glue ear and effect on language development in preschool children: a randomised trial. Lancet 1999;353(9157):960-3. [CENTRAL: CN-00166722] [PMID: ] - PubMed
-
- Maw R, Wilks J, Harvey I, Peters TJ, Golding J, Wiks J. Early surgery compared with watchful waiting for glue ear and effect on language development in preschool children: a randomised trial. Lancet 1999;353(9157):960-3. - PubMed
-
- Maw R, Wilks J, Harvey I, Peters TJ, Golding J. Randomised controlled trial of early surgery versus watchful waiting for glue ear: the effect of langauge development in pre-school children. Journal of Laryngology 1999;113(23 Suppl):40. [CENTRAL: CN-00292554]
-
- Wilks J, Maw R, Peters TJ, Harvey I, Golding J. Randomised controlled trial of early surgery versus watchful waiting for glue ear: the effect on behavioural problems in pre-school children. Clinical Otolaryngology and Allied Sciences 2000;25(3):209-14. [CENTRAL: CN-00330781] [PMID: ] - PubMed
Paradise 2007 {published data only}
-
- Johnston LC, Feldman HM, Paradise JL, Bernard BS, Colborn DK, Casselbrant ML, et al. Tympanic membrane abnormalities and hearing levels at the ages of 5 and 6 years in relation to persistent otitis media and tympanostomy tube insertion in the first 3 years of life: a prospective study incorporating a randomized clinical trial. Pediatrics 2004;114(1):e58-67. [CENTRAL: CN-00490067] [PMID: ] - PubMed
-
- NCT00365092. Middle ear disease before age 3, treatment with ear tubes, and literacy and attentional abilities at ages 9 to 11 [Early otitis and literacy and attention at 9 to 11 years]. clinicaltrials.gov/show/NCT00365092 (first received 15 August 2006). [CENTRAL: CN-02013311]
-
- Paradise J, Dollaghan C, Campbell T, Feldman H. Early vs delayed tube placement for persistent middle-ear effusion in the first three years of life: effects on cognition, language, speech and behavior at ages 4 and 6 years. In: 8th International Symposium on Recent Advances in Otitis Media. Fort Lauderdale, FL, USA, 3-7 June, 2003. 2003:219. [ABSTRACT NO.: B57] [CENTRAL: CN-00449362]
-
- Paradise JL, Colborn DK, Bernard BS, Hakos LM, Guerra NJ, et al. Comparison of the early results of tympanostomy tube placement and of nonsurgical management in infants with persistent otitis media. In: Recent Advances in Otitis Media. 6th International Symposium on Recent Advances in Otitis Media, Jun 04-08, 1995, Ft Lauderdale, Florida. 1996:201. [CENTRAL: CN-00634260]
Popova 2010 {published data only}
-
- Popova D, Varbanova S, Popov TM. Comparison between myringotomy and tympanostomy tubes in combination with adenoidectomy in 3-7-year-old children with otitis media with effusion. International Journal of Pediatric Otorhinolaryngology 2010;74(7):777-80. [CENTRAL: CN-00767591] [PMID: ] - PubMed
Rach 1991 {published data only}
-
- Rach GH, Zielhuis GA, Baarle PW, den Broek P. The effect of treatment with ventilating tubes on language development in preschool children with otitis media with effusion. Clinical Otolaryngology and Allied Sciences 1991;16(2):128-32. [CENTRAL: CN-00076714] [PMID: ] - PubMed
-
- Schilder AGM, Manen JG, Zielhuis GA, Grievink EH. Long-term results of a randomized controlled trial of surgery for otitis media with effusion. In: Sade J , editors(s). Infections in Childhood Ear, Nose and Throat Aspects: Proceedings of the 3rd International Conferenceof the European Working Group for Pediatric Otorhinolargyngology, Jerusalem, Israel: Nov 7-12, 1993. 1994.
-
- Zielhuis GA, Rach GH, den Broek P. Screening for otitis media with effusion in preschool children. Lancet 1989;1(8633):311-4. - PubMed
Rovers 2000 {published data only}
-
- Hartman M, Rovers MM, Ingels K, Zielhuis GA, Severens JL, Wilt GJ. Economic evaluation of ventilation tubes in otitis media with effusion. Archives of Otolaryngology--Head & Neck Surgery 2001;127(12):1471-6. [CENTRAL: CN-00369148] [EMBASE: 2001440522] [PMID: ] - PubMed
-
- Rovers M, Ingels K, Wilt GJ, den Broek P, Zielhuis G. The effects of ventilation tubes: a randomized clinical trial. In: Recent Advances in Otitis Media, Proceedings. 2001:411-4. [CENTRAL: CN-02494418]
-
- Rovers M, Zielhuis G, Hartman M, Ingels K, Straatman H, Van Der Wilt G. The effect of early screening and treatment with ventilation tubes in infants with persistent otitis media with effusion. In: ISTAHC Annual Meeting. Vol. 16. 2000:063. [CENTRAL: CN-00452876]
-
- Rovers MM, Straatman H, Ingels K, Wilt GJ, den Broek P, Zielhuis GA. Generalizability of trial results based on randomized versus nonrandomized allocation of OME infants to ventilation tubes or watchful waiting. Journal of Clinical Epidemiology 2001;54(8):789-94. [CENTRAL: CN-00349653] [PMID: ] - PubMed
Ruckley 1988 {published data only}
-
- Ruckley RW, Blair RL. Thermal myringotomy (an alternative to grommet insertion in childhood secretory otitis media?). Journal of Laryngology and Otology 1988;102(2):125-8. [CENTRAL: CN-00452878] - PubMed
Sujatha 2015 {published data only}
-
- Sujatha S, Venugopalan PG. Evaluation of effectiveness of myringotomy and myringotomy with ventilation tube insertion as treatment of otitis media with effusion in children. Journal of Evolution of Medical and Dental Sciences 2015;4(29):5003-9.
Tao 2020 {published data only}
-
- Tao J, Luo R, Chen Y, Hou C, Hao Q. Myringotomy or tympanostomy tube insertion, comparison of surgical treatment of adenoid hypertrophy and otitis media with effusion in children. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020;34(3):207-10. [DOI: 10.13201/j.issn.2096-7993.2020.03.005] [PMID: ] - DOI - PMC - PubMed
TARGET 2000 {published data only}
-
- Browning G. TARGET trial of alternative regimes for glue ear treatment: analysis 2. In: 8th International Congress of Paediatric Otorhinolaryngology (ESPO). Oxford, UK, 11-14 September, 2002. 2002:60. [CENTRAL: CN-00431582]
-
- Browning GG. Patient-centred oucomes of a surgical RCT of otitis media with effusion at one year. Laryngo-Rhino-Otologie 2000;79(Suppl 1):S35-6. [CENTRAL: CN-00526378]
-
- Browning GG. Two-year outcome of ventilation tubes in a randomized controlled trial of persistent childhood otitis media with effusion. Clinical Otolaryngology and Allied Sciences 2001;26(4):342-4. [CENTRAL: CN-02494417] - PubMed
-
- Haggard MP, Gannon MM, Birkin JA, Bennett KE, Nicholls EE, Spencer H, et al. Adjuvant adenoidectomy in persistent bilateral otitis media with effusion: hearing and revision surgery outcomes through 2 years in the TARGET randomised trial. Clinical Otolaryngology 2012;37(2):107-16. [CENTRAL: CN-00896356] [EMBASE: 364670938] [PMID: ] - PubMed
-
- ISRCTN35793977. Trial of alternative regimens in glue ear treatment - effectiveness of surgery for otitis media with effusion in 3.5-7 year olds using multiple developmental and economic measures combined with classical clinical measures. www.controlled-trials.com/ISRCTN35793977 (first received 2000). [CENTRAL: CN-00725114]
To 1984 {published data only}
-
- To SS, Pahor AL, Robin PE. A prospective study of the use of unilateral grommets in chronic bilateral secretory otitis media. Clinical Otolaryngology and Allied Sciences 1984;9:130-1. [CENTRAL: CN-00262088] - PubMed
-
- To SS, Pahor AL, Robin PE. A prospective trial of unilateral grommets for bilateral secretory otitis media in children. Clinical Otolaryngology and Allied Sciences 1984;9(2):115-7. [CENTRAL: CN-00035295] [PMID: ] - PubMed
Velepic 2011 {published data only}
-
- Velepic M, Starcevic R, Bonifacic M, Ticac R, Kujundzic M, Udovic DS, et al. The clinical status of the eardrum: an inclusion criterion for the treatment of chronic secretory otitis media in children. International Journal of Pediatric Otorhinolaryngology 2011;75(5):686-90. [CENTRAL: CN-00784332] [EMBASE: 51315541] [PMID: ] - PubMed
Yousaf 2016 {published data only}
-
- Yousaf M, Malik SA, Haroon T. Laser myringotomy versus ventilation tubes in otitis media with effusion. Journal of Ayub Medical College, Abbottabad 2016;28(4):773-5. [CENTRAL: CN-01380598] [EMBASE: 619635268] [PMID: ] - PubMed
References to studies excluded from this review
Ah‐Tye 2001 {published data only}
-
- Ah-Tye C, Paradise JL, Colborn DK. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics 2001;107(6):1251-8. [CENTRAL: CN-00348427] [PMID: ] - PubMed
Ardehali 2008 {published data only}
-
- Ardehali MM, Seraj JM, Asiabar MK, Adibi H. The possible role of gastroesophageal reflux disease in children suffering from chronic otitis media with effusion. Acta Medica Iranica 2008;46(1):33-7. [CENTRAL: CN-00708224] [EMBASE: 351792703]
Black 1990 {published data only}
Bozkurt 2004 {published data only}
-
- Bozkurt MK, Calguner M. The efficacy of CO2 laser myringotomy in serous otitis media. Kulak Burun Bogaz Ihtisas Dergisi: KBB [Journal of Ear, Nose, and Throat] 2004;12(3-4):55-9. [CENTRAL: CN-00523128] [PMID: ] - PubMed
Bulman 1984 {published data only}
-
- Bulman CH, Brook SJ, Berry MG. A prospective randomized trial of adenoidectomy vs grommet insertion in the treatment of glue ear. Clinical Otolaryngology and Allied Sciences 1984;9(2):67-75. [CENTRAL: CN-00174952] [EMBASE: 1984176580] [PMID: ] - PubMed
Choung 2008 {published data only}
Demant 2017 {published data only}
-
- Demant MN, Jensen RG, Jakobsen JC, Gluud C, Homoe P. The effects of ventilation tubes versus no ventilation tubes for recurrent acute otitis media or chronic otitis media with effusion in 9 to 36 month old Greenlandic children, the SIUTIT trial: study protocol for a randomized controlled trial. Trials 2017;18(1):30. [CENTRAL: CN-01304999] [EMBASE: 614107422] [PMID: ] - PMC - PubMed
-
- NCT02490332. The effects of ventilation tubes - The SIUTIT Trial [The effects of ventilation tubes versus no ventilation tubes for recurrent acute otitis media or chronic otitis media with effusion in 9 to 36 month old Greenlandic children - a randomised clinical trial]. clinicaltrials.gov/show/NCT02490332 (first received 3 July 2015). [CENTRAL: CN-02043426]
El Begermy 2022 {published data only}
-
- El Begermy MA, El Begermy MM, Kassamy H. The effect of endoscopic peritubal adenoidectomy vs myringotomy with ventilation tubes insertion in management of otitis media with effusion in children. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 2022;23(23):1-10. [CENTRAL: CN-02518322]
Englender 1999 {published data only}
-
- Englender M, Somech E, Harell M. Laser myringotomy (L-myringotomy) and ventilating tubes: a preliminary comparative study. Lasers in Medical Science 1999;14(1):62-6. [CENTRAL: CN-00431622] - PubMed
Ferrara 2005 {published data only}
-
- Ferrara S, Sammartano D, Ferrara P. Long-term management of recurrent otitis media with effusion in children. In: XVIII IFOS World Congress; 2005 Jun 25-30, Rome (Italy). 2005. [CENTRAL: CN-00526409]
Gebhart 1981 {published data only}
-
- Gebhart DE. Tympanostomy tubes in the otitis media prone child. Laryngoscope 1981;91(6):849-66. [CENTRAL: CN-00025323] [EMBASE: 1981143267] [PMID: ] - PubMed
Gibson 1996 {published data only}
-
- Gibson PG, Stuart JE, Wlodarczyk J, Olson LG, Hensley MJ. Nasal inflammation and chronic ear disease in Australian Aboriginal children. Journal of Paediatrics and Child Health 1996;32(2):143-7. [CENTRAL: CN-00131589] [PMID: ] - PubMed
Hammaren‐Malmi 2005 {published data only}
-
- Hammaren-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics 2005;116(1):185-9. [CENTRAL: CN-00528797] [PMID: ] - PubMed
Hao 2019 {published data only}
-
- Hao J, Chen M, Liu B, Yang Y, Liu W, Ma N, et al. Compare two surgical interventions for otitis media with effusion in young children. European Archives of Oto-rhino-laryngology 2019;276(8):2125-31. [CENTRAL: CN-01956237] [EMBASE: 627898353] [PMID: ] - PubMed
-
- Hao J, Chen M, Liu B, Yang Y, Liu W, Ma N, et al. Correction to: compare two surgical interventions for otitis media with effusion in young children [Correction to: Compare two surgical interventions for otitis media with effusion in young children (European Archives of Oto-Rhino-Laryngology, (2019), 276, 8, (2125-2131), 10.1007/s00405-019-05421-9)]. European Archives of Oto-rhino-laryngology 2019;276(8):2133-4. [CENTRAL: CN-02080898] [EMBASE: 628234545] [PMID: ]
Hassmann 2004 {published data only}
-
- Hassmann E, Skotnicka B, Baczek M, Piszcz M. Laser myringotomy in otitis media with effusion: long-term follow-up. European Archives of Oto-rhino-laryngology 2004;261(6):316-20. [CENTRAL: CN-00497408] - PubMed
Iino 1989 {published data only}
-
- Iino Y, Ishitoya J, Ikeda M, Ito Y, Usami M, Kawashiro N, et al. Factors on delayed recovery of otitis media with effusion in children--clinical and bacteriological study. Nihon Jibiinkoka Gakkai Kaiho 1989;92(8):1183-91. [CENTRAL: CN-00063873] [PMID: ] - PubMed
Jabeen 2019 {published data only}
-
- Jabeen F, Chaudhry S, Ahmed Z, Khalil N, Amin B, Rizvi SS. Comparison of rate of recurrence of otitis media with effusion in children treated by myringotomy and ventilating tube insertion with patients treated by additional adenoidectomy. Rawal Medical Journal 2019;44(3):513-6. [CENTRAL: CN-02007003] [EMBASE: 2002854226]
Kremer 1979 {published data only}
-
- Kremer M, Podoshin L, Fradis M. Treatment of serous otitis media with tympanic ventilation tubes. Ear, Nose, & Throat Journal 1979;58(5):203-9. [PMID: ] - PubMed
Kujala 2012 {published data only}
-
- Kujala T, Alho OP, Kristo A, Uhari M, Renko M, Pokka T, et al. Quality of life after surgery for recurrent otitis media in a randomized controlled trial. Pediatric Infectious Disease Journal 2014;33(7):715-9. [CENTRAL: CN-00995945] [EMBASE: 2014406374] [PMID: ] - PubMed
Li 2020 {published data only}
-
- Li H, Zhou Y, Huang J, Zhang L, Lou Z. Treatment of secretory otitis media with balloon dilation and tympanostomy tube insertion: a randomized controlled trial. Basic & Clinical Pharmacology & Toxicology 2020;127(Suppl 1):292-3. [CENTRAL: CN-02230226] [EMBASE: 633830686]
Lildholdt 1983 {published data only}
-
- Lildholdt T. Ventilation tubes in secretory otitis media. A randomized, controlled study of the course, the complications, and the sequelae of ventilation tubes. Acta Oto-laryngologica. Supplementum 1983;398:1-28. [CENTRAL: CN-00033048] [EMBASE: 1984015453] [PMID: ] - PubMed
Liu 2004 {published data only}
-
- Liu L, Sun YG, Ma L, Zhao W, Wu R. Effect of ventilation tube insertion on otitis media with effusion in cleft palate children. Zhonghua Er Bi Yan Hou Ke za Zhi 2004;39(4):216-8. [CENTRAL: CN-00549233] [EMBASE: 39714817] - PubMed
Mandel 1989 {published data only}
-
- Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Myringotomy with and without tympanostomy tubes for chronic otitis media with effusion. Archives of Otolaryngology--Head & Neck Surgery 1989;115(10):1217-24. [CENTRAL: CN-00062654] [PMID: ] - PubMed
Mandel 1992 {published data only}
-
- Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Efficacy of myringotomy with and without tympanostomy tubes for chronic otitis media with effusion. Pediatric Infectious Disease Journal 1992;11(4):270-7. [CENTRAL: CN-00338034] [PMID: ] - PubMed
Marchisio 1998 {published data only}
-
- Marchisio P, Principi N, Passali D, Salpietro DC, Boschi G, Chetri G, et al. Epidemiology and treatment of otitis media with effusion in children in the first year of primary school. Acta Oto-laryngologica 1998;118(4):557-62. [CENTRAL: CN-00154484] [EMBASE: 1998254893] [PMID: ] - PubMed
Markou 2004 {published data only}
-
- Markou K, Vlachtsis K, Kyriafinis G, Petridis D, Daniilidis I. Long-term results of adenoidectomy and ventilation tube insertion in the surgical treatment of chronic otitis media with effusion. International Journal of Pediatric Otorhinolaryngology 2004;68(5):684. [CENTRAL: CN-00477460]
Maw 1993 {published data only}
Moller 1990 {published data only}
-
- Moller P, Dingsor G. Otitis media with effusion: can erythromycin reduce the need for ventilating tubes? Journal of Laryngology and Otology 1990;104(3):200-2. [CENTRAL: CN-00067688] [PMID: ] - PubMed
MRC Multicentre Otitis Media Study 2004 {published data only}
-
- MRC Multicentre Otitis Media Study Group. Speech reception in noise: an indicator of benefit from otitis media with effusion surgery. Clinical Otolaryngology and Allied Sciences 2004;29(5):497-504. [CENTRAL: CN-00497404] [EMBASE: 39287082] [PMID: ] - PubMed
MRC Multicentre Otitis Media Study 2008 {published data only}
-
- MRC Multicentre Otitis Media Study Group. An extension of the Jerger classification of tympanograms for ventilation tube patency--specification and evaluation of equivalent ear-canal volume criteria. Ear and Hearing 2008;29(6):894-906. [CENTRAL: CN-00682310] [EMBASE: 550156802] [PMID: ] - PubMed
NCT00629694 {published data only}
-
- NCT00629694. Adenoidectomy, myringotomy and tubes' insertion vs adenoidectomy and myringotomy alone in children with otitis media with effusion and adenoid hypertrophy. clinicaltrials.gov/show/NCT00629694 (first received 6 March 2008). [CENTRAL: CN-00726812]
NCT05545345 {published data only}
-
- NCT05545345. Adjuvant adenoidectomy for the treatment of chronic OME in children [Adjuvant adenoidectomy plus tympanostomy tube placement for the treatment of chronic OME in children]. clinicaltrials.gov/show/NCT05545345 (first received 19 September 2022). [CENTRAL: CN-02462316]
Nguyen 2004 {published data only}
-
- Nguyen LH, Manoukian JJ, Yoskovitch A, Al-Sebeih KH, Al Sebeih KH. Adenoidectomy: selection criteria for surgical cases of otitis media. Laryngoscope 2004;114(5):863-6. [CENTRAL: CN-00469074] [PMID: ] - PubMed
Paradise 1990 {published data only}
-
- Paradise JL, Bluestone CD, Rogers KD, Taylor FH, Colborn DK, Bachman RZ, et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement. Results of parallel randomized and nonrandomized trials. JAMA 1990;263(15):2066-73. [CENTRAL: CN-00066665] [PMID: ] - PubMed
Paradise 1997 {published data only}
-
- Paradise J, Campbell T, Dollaghan C, Feldman H, Bernard B, Colborn K, et al. Receptive vocabulary, cognition, and parent-rated behavior at age 3 years in relation to otitis media in the first 3 years of life. In: Abstract Book of the Association of Health Service Research. Vol. 14. 1997:350-1. [CENTRAL: CN-00452820]
Parlea 2012 {published data only}
Rohail 2006 {published data only}
-
- Rohail A, Gill ZI, Butt MR. A comparison of medical treatment versus surgical treatment for the management of otitis media with effusion. Annals of King Edward Medical College 2006;12(1):64-7. [CENTRAL: CN-00597368]
Sanyaolu 2020 {published data only}
-
- Sanyaolu LN, Cannings-John R, Butler CC, Francis NA. The effect of ventilation tube insertion on quality of life in children with persistent otitis media with effusion. Clinical Otolaryngology 2020;45(2):239-47. [CENTRAL: CN-02217143] [EMBASE: 2004053181] [PMID: ] - PubMed
Shishegar 2007 {published data only}
-
- Shishegar M, Hoghoghi H. Comparison of adenoidectomy and myringotomy with and without tube placement in the short term hearing status of children with otitis media with effusion: a preliminary report. Iranian Journal of Medical Sciences 2007;32(3):169-72. [CENTRAL: CN-00708699] [EMBASE: 351270400]
Shubich 1996 {published data only}
-
- Shubich I. Otitis media with effusion and allergy control in children: a prospective study. In: Sixth International Symposium on Otitis Media; 1996, Fort Lauderdale (FL). 1996:173-4. [CENTRAL: CN-00452904]
Skinner 1988 {published data only}
-
- Skinner DW, Lesser TH, Richards SH. A 15-year follow-up of a controlled trial of the use of grommets in glue ear. Otolaryngology and Allied Sciences 1989;14:169. - PubMed
-
- Skinner DW, Lesser THJ, Richards SH. A 15 year follow-up of a controlled trial of the use of grommets in glue ear. Clinical Otolaryngology and Allied Sciences 1988;13(5):341-6. [EMBASE: 18269160] - PubMed
Stenstrom 2005 {published data only}
-
- Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Archives of Pediatrics & Adolescent Medicine 2005;159(12):1151-6. [CENTRAL: CN-00532329] [PMID: ] - PubMed
Tao 2004 {published data only}
-
- Tao LH, He D-H, Huang Y-D. Effects of adenoidectomy in treatment of secretory otitis media in children. Journal of Clinical Pediatric Surgery 2004;4:253-5. [CENTRAL: CN-00849652]
Uvarova 2001 {published data only}
-
- Uvarova N. Comparison of the results of treatment of otitis media with effusion after myringotomy with and without ventiliation tube. In: 4th Extraordinary International Symposium on Recent Advances in Otitis Media; 2001 Apr 16-20, Sendai (Japan). 2001:165. [ABSTRACT NO.: 122] [CENTRAL: CN-00362633]
Xu 2016 {published data only}
-
- Xu WM, Ye YH. Effect of tympanostomy tube insertion with adenoidectomy for children with recurrent otitis media with effusion. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2016;30(23):1873-5. [CENTRAL: CN-01614396] [EMBASE: 622451696] [PMID: ] - PubMed
Yousaf 2014 {published data only}
-
- Yousaf M, Malik SA, Zada B. Laser and incisional myringotomy in otitis media with effusion-a comparative study. Journal of Ayub Medical College, Abbottabad 2014;26(4):441-3. [CENTRAL: CN-01043373] [PMID: ] - PubMed
Youssef 2013 {published data only}
References to studies awaiting assessment
Diacova 2016 {published data only}
-
- Diacova S, McDonald TJ, Ababii I. Clinical, functional, and surgical findings in chronic bilateral otitis media with effusion in childhood. Ear, Nose, & Throat Journal 2016;95(8):E31-7. [CENTRAL: CN-01339417] [EMBASE: 614412861] [PMID: ] - PubMed
Marshak 1980 {published data only}
-
- Marshak G, Neriah ZB. Adenoidectomy versus tympanostomy in chronic secretory otitis media. Annals of Otology, Rhinology, and Laryngology. Supplement 1980;89(3 Part 2):316-8. - PubMed
Maw 1986 {published data only}
-
- Maw AR. Adenoidectomy and adenotonsillectomy for otitis media with effusion in children: a prospective randomized controlled study (Masters thesis). Bristol, UK: University of Bristol, 1986. [CENTRAL: CN-00849155]
Tawfik 2002 {published data only}
-
- Tawfik S, Belal A, Sorour W. A comparative study of the different treatment modalities of otitis media with effusion in children. In: 8th International Congress of Paediatric Otorhinolaryngology (ESPO). Oxford, UK, 11-14 September, 2002. 2002:151. [ABSTRACT NUMBER: P2.26] [CENTRAL: CN-00508402]
References to ongoing studies
ACTRN12611001073998 {unpublished data only}
-
- ACTRN12611001073998. Surgery for otitis media in Indigenous Australian children [A 12 month, multi-centred, randomized trial to compare the outcomes of two surgical and one medical intervention on chronic Otitis Media in Indigenous children living in remote communities of Australia. Medicine V surgery sub-study]. www.anzctr.org.au/ACTRN12611001073998.aspx (first received 17 October 2011). [CENTRAL: CN-01012938]
NCT02546518 {published data only}
-
- NCT02546518. A comparison of surgical and a new non-surgical treatment methods for secretory otitis media in children- hearing and socioeconomic aspects. clinicaltrials.gov/show/nct02546518 (first received 11 September 2015). [CENTRAL: CN-01102072]
NCT04584073 {published data only}
-
- NCT04584073. Secretory otitis media in adenoids hypertrophy patients [Comparison between the fate of secretory otitis media in patients with adenoids hypertrophy undergoing adenoidectomy alone or with myringotomy or with myringotomy and tympanostomy tube application]. clinicaltrials.gov/show/NCT04584073 (first received 12 October 2020). [CENTRAL: CN-02181953]
Additional references
Abidin 1995
-
- Abidin RR. Parenting Stress Index: Professional Manual. 3rd edition. Odessa, FL: Psychological Assessment Resources, 1995.
Achenbach 2011
-
- Achenbach TM. Child Behavior Checklist. In: Kreutzer JS, DeLuca J, Caplan B, editors(s). Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 2011. [DOI: 10.1007/978-0-387-79948-3_1529] - DOI
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, Inc, 2006.
Bennett 1999
Bennett 2001
Berkman 2013
-
- Berkman ND, Wallace IF, Steiner MJ, Harrison M, Greenblatt AM, Lohr KN, et al. Otitis Media with Effusion: Comparative Effectiveness of Treatments. Vol. Report No.: 13-EHC091-EF. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. [PMID: ] - PubMed
Browning 2010
Bruce 2015
Cheong 2012
-
- Cheong KH, Hussain SS. Management of recurrent acute otitis media in children: systematic review of the effect of different interventions on otitis media recurrence, recurrence frequency and total recurrence time. Journal of Laryngology & Otology 2012;126(9):874-85. - PubMed
Cochrane ENT 2020
-
- Cochrane ENT. Otitis media with effusion: a project to prioritise Cochrane systematic reviews. https://ent.cochrane.org/otitis-media-effusion-ome-glue-ear 2020 (accessed 3 November 2021).
de Beer 2004
de Beer 2005
Dunn 2007
-
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test (PPVT™-4). 4th edition. Pearson Education, 2007.
Fekkes 2000
Flynn 2009
Galbraith 2023
Goodman 1997
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 27 February 2023. Available at gradepro.org.
Gresham 1990
-
- Gresham FM, Elliott SN. Social Skills Rating System. Circle Pines, MN: American Guidance Service, 1990.
Griffiths 1996
-
- Griffiths R. The Griffiths mental development scales from birth to two years, manual, the 1996 revision. Henley: Association for Research in Infant and Child Development, Test Agency, 1996.
Haggard 2003
-
- Haggard MP, Smith SC, Nicholls EE. Quality of life and child behaviour. In: Rosenfeld RM, Bluestone CD, editors(s). Evidence-based Otitis Media. 2nd edition. Hamilton, Ontario: BC Decker Inc, 2003:401-29. [https://researchonline.lshtm.ac.uk/id/eprint/15108]
Hedrick 1984
-
- Hedrick DL, Prather EM, Tobin AR. Sequenced Inventory of Communication Development. Seattle, WA: University of Washington Press, 1984.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Jellinek 1988
Kreiner‐Møller 2012
Laina 2006
Landgraf 1994
-
- Landgraf JM. The Infant/Toddler Child Health Questionnaire: conceptual framework, logic content, and preliminary psychometric results. Boston: Health Act, 1994.
Landgraf 1996
-
- Landgraf JL, Abetz L, Ware JE. The CHQ User’s Manual. Boston: The Health Institute, New England Medical Center, 1996.
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Liu 2020
-
- Liu PZ, Ismail-Koch H, Stephenson K, Donne AJ, Fergie N, Derry J, et al. A core outcome set for research on the management of otitis media with effusion in otherwise-healthy children. International Journal of Pediatric Otorhinolaryngology 2020 ;134:Article 110029. [DOI: 10.1016/j.ijporl.2020.110029] - DOI - PubMed
MacKeith 2023
Maris 2014
Marseglia 2008
Marshall 2018
McCarthy 1972
-
- McCarthy D. Manual for the McCarthy Scales of Children's Abilities. New York: Psychological Corp, 1972.
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-17; Cape Town, South Africa. 2017.
Mulvaney 2023a
Mulvaney 2023b
NICE 2008
-
- NICE. Otitis media with effusion in under 12s: Otitis media with effusion in under 12s: surgery (CG60). Available at: https://www.nice.org.uk/guidance/cg60 2008.
NICE CKS 2021
-
- NICE. Otitis media with effusion (Clinical Knowledge Summary). Available at: https://cks.nice.org.uk/topics/otitis-media-with-effusion/ 2021.
Noel‐Storr 2018
-
- Noel-Storr AH. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Rabin 2001
RevMan 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Reynell 1985
-
- Reynell JH. Reynell Development Language Scales Manual. 2nd edition. Windsor, UK: NFER-NELSON, 1985.
Rosenfeld 1997
Rosenfeld 2000
-
- Rosenfeld RM, Bhaya MH, Bower CM, Brookhouser PE, Casselbrant ML, Chan KH, et al. Impact of tympanostomy tubes on child quality of life. Archives of Otolaryngology - Head & Neck Surgery 2000;126:585-92. - PubMed
Rosenfeld 2003
Rosenfeld 2016
Schilder 2016
Schlichting 2007
-
- Schlichting JEPT, Lutje Spelberg HC. Lexilijst Begrip: An Instrument to Investigate Language Comprehension in Children aged 15-25 Months in the Context of Early Identification. Amsterdam: Pearson Assessment & Information BV, 2007.
Schlichting 2010
-
- Schlichting JEPT, Lutje Spelberg HC. Schlichting Test for Language Comprehension; Instruction Manual. Woooden: Bohn Stafleu van Loghum, 2010.
Schmalbach 2021
-
- Schmalbach CE, Brereton J, Bowman C, Denneny JC. American Academy of Otolaryngology–Head and Neck Surgery/Foundation Reg-ent Registry: Purpose, Properties, and Priorities. Otolaryngology–Head and Neck Surgery 2021;164(5):964-71. - PubMed
Steele 2017
-
- Steele D, Adam GP, Di M, Halladay C, Pan I, Coppersmith N, et al. Tympanostomy tubes in children with otitis media. Comparative Effectiveness Review No. 185 (Prepared by the Brown Evidence-based Practice Center under Contract No. 290-2015-00002-I.). AHRQ Publication No. 17-EHC003-EF. Rockville, MD: Agency for Healthcare Research and Quality, 2017. [DOI: 10.23970/AHRQEPCCER185] - DOI - PubMed
Thomas 2017
TNO 1997
-
- TNO—Prevention and Health/LUMC. TAIQOL—Questionnaire for parents of children aged 1—5 years. Leiden, The Netherlands: Leiden University Medical Center, 1997.
van den Aardweg 2010
Vanneste 2019
Venekamp 2018
Verrips 1998
-
- Verrips GH, Vogels AGC, Verloove-Vanhorick SP, Fekkes M, Koopman HM, Kamphuis RP, et al. Health-related quality of life measure for children - the TACQOL. Journal of Applied Therapeutics 1998;1(4):357-60.
Wallace 2014
Wallace 2017
Williamson 2011
Zernotti 2017
Zielhuis 1990
-
- Zielhaus GA, Rach GH, den Broek P. The occurrence of otitis media with effusion in Dutch pre-school children. Clinical Otolaryngology & Allied Sciences 1990;15(2):147-53. - PubMed
Zimmermann 1992
-
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-3. San Antonio, TX: The Psychological Corporation, 1992.
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

