Intratumoral androgen biosynthesis associated with 3β-hydroxysteroid dehydrogenase 1 promotes resistance to radiotherapy in prostate cancer
- PMID: 37966114
- PMCID: PMC10645386
- DOI: 10.1172/JCI165718
Intratumoral androgen biosynthesis associated with 3β-hydroxysteroid dehydrogenase 1 promotes resistance to radiotherapy in prostate cancer
Abstract
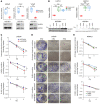
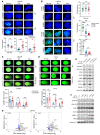
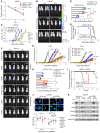
Half of all men with advanced prostate cancer (PCa) inherit at least 1 copy of an adrenal-permissive HSD3B1 (1245C) allele, which increases levels of 3β-hydroxysteroid dehydrogenase 1 (3βHSD1) and promotes intracellular androgen biosynthesis. Germline inheritance of the adrenally permissive allele confers worse outcomes in men with advanced PCa. We investigated whether HSD3B1 (1245C) drives resistance to combined androgen deprivation and radiotherapy. Adrenally permissive 3βHSD1 enhanced resistance to radiotherapy in PCa cell lines and xenograft models engineered to mimic the human adrenal/gonadal axis during androgen deprivation. The allele-specific effects on radiosensitivity were dependent on availability of DHEA, the substrate for 3βHSD1. In lines expressing the HSD3B1 (1245C) allele, enhanced expression of DNA damage response (DDR) genes and more rapid DNA double-strand break (DSB) resolution were observed. A correlation between androgen receptor (AR) expression and increased DDR gene expression was confirmed in 680 radical prostatectomy specimens. Treatment with the nonsteroidal antiandrogen enzalutamide reversed the resistant phenotype of HSD3B1 (1245C) PCa in vitro and in vivo. In conclusion, 3βHSD1 promotes prostate cancer resistance to combined androgen deprivation and radiotherapy by upregulating DNA DSB repair. This work supports prospective validation of early combined androgen blockade for high-risk men harboring the HSD3B1 (1245C) allele.
Keywords: Endocrinology; Prostate cancer; Radiation therapy; Sex hormones; Therapeutics.
Conflict of interest statement
Figures


References
-
- Hanks GE, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21(21):3972–3978. doi: 10.1200/JCO.2003.11.023. - DOI - PubMed
-
- Krauss DJ, et al. Dose escalated radiotherapy alone or in combination with short-term androgen suppression for intermediate risk prostate cancer: outcomes from the NRG oncology/RTOG 0815 Randomized Trial. Int J Radiat Oncol Biol Phys. 2021;111(3):S1. doi: 10.1016/j.ijrobp.2021.07.039. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

