Essential role of the conserved oligomeric Golgi complex in Toxoplasma gondii
- PMID: 37966241
- PMCID: PMC10746232
- DOI: 10.1128/mbio.02513-23
Essential role of the conserved oligomeric Golgi complex in Toxoplasma gondii
Abstract
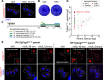
The Golgi is an essential eukaryotic organelle and a major place for protein sorting and glycosylation. Among apicomplexan parasites, Toxoplasma gondii retains the most developed Golgi structure and produces many glycosylated factors necessary for parasite survival. Despite its importance, Golgi function received little attention in the past. In the current study, we identified and characterized the conserved oligomeric Golgi complex and its novel partners critical for protein transport in T. gondii tachyzoites. Our results suggest that T. gondii broadened the role of the conserved elements and reinvented the missing components of the trafficking machinery to accommodate the specific needs of the opportunistic parasite T. gondii.
Keywords: AP-5; COPI; COPII; Golgi; Toxoplasma gondii; anterograde transport; apicomplexa; glycosylation; retrograde transport; vesicular transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

