Perspectives of cyanobacterial cell factories
- PMID: 37966575
- PMCID: PMC11615099
- DOI: 10.1007/s11120-023-01056-4
Perspectives of cyanobacterial cell factories
Abstract
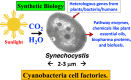
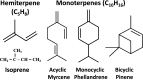
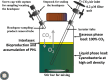
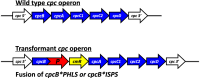
Cyanobacteria are prokaryotic photosynthetic microorganisms that can generate, in addition to biomass, useful chemicals and proteins/enzymes, essentially from sunlight, carbon dioxide, and water. Selected aspects of cyanobacterial production (isoprenoids and high-value proteins) and scale-up methods suitable for product generation and downstream processing are addressed in this review. The work focuses on the challenge and promise of specialty chemicals and proteins production, with isoprenoid products and biopharma proteins as study cases, and the challenges encountered in the expression of recombinant proteins/enzymes, which underline the essence of synthetic biology with these microorganisms. Progress and the current state-of-the-art in these targeted topics are emphasized.
Keywords: Synechocystis sp. PCC 6803; Biopharmaceutical proteins; Fusion constructs; Phycocyanin; Plant essential oils; Protein overexpression.
© 2023. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests.
Figures


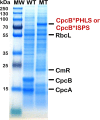
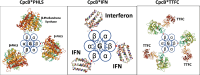
References
-
- Albers SC, Gallegos VA, Peebles CAM (2015) Engineering of genetic control tools in Synechocystis sp. PCC 6803 using rational design techniques. J Biotechnol 216:36–46. 10.1016/j.jbiotec.2015.09.042 - PubMed
-
- Bäck J, Aaltonen H, Hellén H, Kajos MJ, Patokoski J, Taipale R, Pumpanen J, Heinonsalo J (2010) Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos Environ 44:3651–3659. 10.1016/j.atmosenv.2010.06.042
-
- Baier T, Kros D, Feiner RC, Lauersen KJ, Müller KM, Kruse O (2018) Engineered fusion proteins for efficient protein secretion and purification of a human growth factor from the green microalga Chlamydomonas reinhardtii. ACS Synth Biol 7:2547–2557. 10.1021/acssynbio.8b00226 - PubMed
-
- Barone GD, Cernava T, Ullmann J, Liu J, Lio E, Germann AT, Nakielski A, Russo DA, Chavkin T, Knufmann K, Tripodi F, Coccetti P, Secundo F, Fu P, Pfleger B, Axmann IM, Lindblad P (2023) Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 9(4):e14708. 10.1016/j.heliyon.2023.e14708 - PMC - PubMed
-
- Behler J, Vijay D, Hess WR, Akhtar MK (2018) CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol 36(10):996–1010. 10.1016/j.tibtech.2018.05.011 - PubMed

