Transcriptome analysis of peripheral blood of Schistosoma mansoni infected children from the Albert Nile region in Uganda reveals genes implicated in fibrosis pathology
- PMID: 37967122
- PMCID: PMC10686515
- DOI: 10.1371/journal.pntd.0011455
Transcriptome analysis of peripheral blood of Schistosoma mansoni infected children from the Albert Nile region in Uganda reveals genes implicated in fibrosis pathology
Abstract
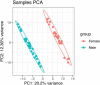
Over 290 million people are infected by schistosomes worldwide. Schistosomiasis control efforts focus on mass drug treatment with praziquantel (PZQ), a drug that kills the adult worm of all Schistosoma species. Nonetheless, re-infections have continued to be detected in endemic areas with individuals living in the same area presenting with varying infection intensities. Our objective was to characterize the transcriptome profiles in peripheral blood of children between 10-15 years with varying intensities of Schistosoma mansoni infection living along the Albert Nile in Uganda. RNA extracted from peripheral blood collected from 44 S. mansoni infected (34 high and 10 low by circulating anodic antigen [CAA] level) and 20 uninfected children was sequenced using Illumina NovaSeq S4 and the reads aligned to the GRCh38 human genome. Differential gene expression analysis was done using DESeq2. Principal component analysis revealed clustering of gene expression by gender when S. mansoni infected children were compared with uninfected children. In addition, we identified 14 DEGs between S. mansoni infected and uninfected individuals, 56 DEGs between children with high infection intensity and uninfected individuals, 33 DEGs between those with high infection intensity and low infection intensity and no DEGs between those with low infection and uninfected individuals. We also observed upregulation and downregulation of some DEGs that are associated with fibrosis and its regulation. These data suggest expression of fibrosis associated genes as well as genes that regulate fibrosis in S. mansoni infection. The relatively few significant DEGS observed in children with schistosomiasis suggests that chronic S. mansoni infection is a stealth infection that does not stimulate a strong immune response.
Copyright: © 2023 Namulondo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2019 –Schistosomiase et géohelminthiases: nombre de personnes traitées en 2019. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire. 2020;95: 629–640. Available: https://apps.who.int/iris/handle/10665/337573
-
- Tukahebwa EM, Magnussen P, Madsen H, Kabatereine NB, Nuwaha F, Wilson S, et al.. A Very High Infection Intensity of Schistosoma mansoni in a Ugandan Lake Victoria Fishing Community Is Required for Association with Highly Prevalent Organ Related Morbidity. PLoS Negl Trop Dis. 2013;7: e2268. doi: 10.1371/journal.pntd.0002268 - DOI - PMC - PubMed
-
- Mulindwa J, Namulondo J, Kitibwa A, Nassuuna J, Nyangiri OA, Kimuda MP, et al.. High prevalence of Schistosoma mansoni infection and stunting among school age children in communities along the Albert-Nile, Northern Uganda: A cross sectional study. WEBSTER JP, editor. PLoS Negl Trop Dis. 2022;16: e0010570. doi: 10.1371/journal.pntd.0010570 - DOI - PMC - PubMed
-
- Exum NG, Kibira SPS, Ssenyonga R, Nobili J, Shannon AK, Ssempebwa JC, et al.. The prevalence of schistosomiasis in Uganda: A nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS Negl Trop Dis. 2019/08/15. 2019;13: e0007617. doi: 10.1371/journal.pntd.0007617 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

