Detection of Recurrence through microRNA-371a-3p Serum Levels in a Follow-up of Stage I Testicular Germ Cell Tumors in the DRKS-00019223 Study
- PMID: 37967143
- PMCID: PMC10792362
- DOI: 10.1158/1078-0432.CCR-23-0730
Detection of Recurrence through microRNA-371a-3p Serum Levels in a Follow-up of Stage I Testicular Germ Cell Tumors in the DRKS-00019223 Study
Abstract
Purpose: Surveillance of clinical stage I (CSI) testicular germ cell tumors (GCT) is hampered by low sensitivity and specificity of current biomarkers for detecting relapses. This study evaluated if serum levels of microRNA371a-3p (M371 test) can: (i) Accurately detect relapses, (ii) detect relapses earlier than conventional technology, and (iii) if elevated postoperative M371 levels may predict relapse.
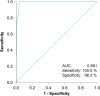
Experimental design: In a multicentric setting, 258 patients with testicular CSI GCT were prospectively followed by surveillance for a median time of 18 months with serial measurements of serum M371 levels, in addition to standard diagnostic techniques. Diagnostic characteristics of M371 for detecting relapses were calculated using ROC curve analysis.
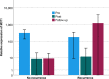
Results: Thirty-nine patients recurred (15.1%), all with elevated M371 levels; eight without relapse had elevations, too. The test revealed the following characteristics: area under the ROC curve of 0.993, sensitivity 100%, specificity 96.3%, positive predictive value 83%, negative predictive value 100%. Earlier relapse detection with the test was found in 28%, with non-significant median time gain to diagnosis. Postoperative M371 levels did not predict future relapse.
Conclusions: The sensitivity and specificity of the M371 test for detecting relapses in CSI GCTs are much superior to those of conventional diagnostics. However, post-orchiectomy M371 levels are not predictive of relapse, and there is no significant earlier relapse detection with the test. In all, there is clear evidence for the utility of the M371 test for relapse detection suggesting it may soon be ready for implementation into routine follow-up schedules for patients with testicular GCT.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ 2022;379:e070499. - PubMed
-
- Zengerling F, Kunath F, Jensen K, Ruf C, Schmidt S, Spek A. Prognostic factors for tumor recurrence in patients with clinical stage I seminoma undergoing surveillance-a systematic review. Urol Oncol 2018;36:448–58. - PubMed
-
- Blok JM, Pluim I, Daugaard G, Wagner T, Jóźwiak K, Wilthagen EA, et al. . Lymphovascular invasion and presence of embryonal carcinoma as risk factors for occult metastatic disease in clinical stage I nonseminomatous germ cell tumor: a systematic review and meta-analysis. BJU Int 2019;124:424–30. - PMC - PubMed
-
- Oldenburg J, Berney DM, Bokemeyer C, Climent MA, Daugaard G, Gietema JA, et al. . Testicular seminoma and nonseminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up. Ann Oncol 2022;33:362–75. - PubMed
-
- Hudolin T, Kastelan Z, Knezevic N, Goluza E, Tomas D, Coric M. Correlation between retroperitoneal lymph node size and presence of metastases in nonseminomatous germ cell tumors. Int J Surg Pathol 2012;20:15–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

