Promotion of uterine reconstruction by a tissue-engineered uterus with biomimetic structure and extracellular matrix microenvironment
- PMID: 37967178
- PMCID: PMC10651121
- DOI: 10.1126/sciadv.adi6488
Promotion of uterine reconstruction by a tissue-engineered uterus with biomimetic structure and extracellular matrix microenvironment
Abstract
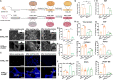
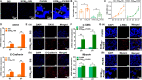
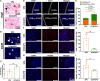
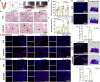
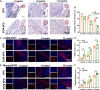
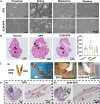
The recurrence rate for severe intrauterine adhesions is as high as 60%, and there is still lack of effective prevention and treatment. Inspired by the nature of uterus, we have developed a bilayer scaffold (ECM-SPS) with biomimetic heterogeneous features and extracellular matrix (ECM) microenvironment of the uterus. As proved by subtotal uterine reconstruction experiments, the mechanical and antiadhesion properties of the bilayer scaffold could meet the requirement for uterine repair. With the modification with tissue-specific cell-derived ECM, the ECM-SPS had the ECM microenvironment signatures of both the endometrium and myometrium and exhibited the property of inducing stem cell-directed differentiation. Furthermore, the ECM-SPS has recruited more endogenous stem cells to promote endometrial regeneration at the initial stage of repair, which was accompanied by more smooth muscle regeneration and a higher pregnancy rate. The reconstructed uterus could also sustain normal pregnancy and live birth. The ECM-SPS may thereby provide a potential treatment for women with severe intrauterine adhesions.
Figures


References
-
- Salazar C. A., Isaacson K., Morris S., A comprehensive review of Asherman’s syndrome: Causes, symptoms and treatment options. Curr. Opin. Obstet. Gynecol. 29, 249–256 (2017). - PubMed
-
- Zhang X., Liu W., Zhou Y., Qiu J., Sun Y., Li M., Ding Y., Xi Q., Comparison of therapeutic efficacy of three methods to prevent re-adhesion after hysteroscopic intrauterine adhesion separation: A parallel, randomized and single-center trial. Ann. Palliat. Med. 10, 6804–6823 (2021). - PubMed
-
- Yin Z., Wang J., Cui W., Tong C., Advanced biomaterials for promoting endometrial regeneration. Adv. Healthc. Mater. 12, e2202490 (2023). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

