Centromere innovations within a mouse species
- PMID: 37967185
- PMCID: PMC10651114
- DOI: 10.1126/sciadv.adi5764
Centromere innovations within a mouse species
Abstract
Mammalian centromeres direct faithful genetic inheritance and are typically characterized by regions of highly repetitive and rapidly evolving DNA. We focused on a mouse species, Mus pahari, that we found has evolved to house centromere-specifying centromere protein-A (CENP-A) nucleosomes at the nexus of a satellite repeat that we identified and termed π-satellite (π-sat), a small number of recruitment sites for CENP-B, and short stretches of perfect telomere repeats. One M. pahari chromosome, however, houses a radically divergent centromere harboring ~6 mega-base pairs of a homogenized π-sat-related repeat, π-satB, that contains >20,000 functional CENP-B boxes. There, CENP-B abundance promotes accumulation of microtubule-binding components of the kinetochore and a microtubule-destabilizing kinesin of the inner centromere. We propose that the balance of pro- and anti-microtubule binding by the new centromere is what permits it to segregate during cell division with high fidelity alongside the older ones whose sequence creates a markedly different molecular composition.
Figures
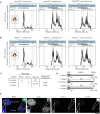





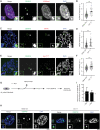
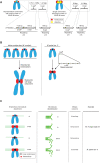
Update of
-
Centromere Innovations Within a Mouse Species.bioRxiv [Preprint]. 2023 May 13:2023.05.11.540353. doi: 10.1101/2023.05.11.540353. bioRxiv. 2023. Update in: Sci Adv. 2023 Nov 17;9(46):eadi5764. doi: 10.1126/sciadv.adi5764. PMID: 37333154 Free PMC article. Updated. Preprint.
References
-
- N. Altemose, G. A. Logsdon, A. V. Bzikadze, P. Sidhwani, S. A. Langley, G. V. Caldas, S. J. Hoyt, L. Uralsky, F. D. Ryabov, C. J. Shew, M. E. G. Sauria, M. Borchers, A. Gershman, A. Mikheenko, V. A. Shepelev, T. Dvorkina, O. Kunyavskaya, M. R. Vollger, A. Rhie, A. M. McCartney, M. Asri, R. Lorig-Roach, K. Shafin, J. K. Lucas, S. Aganezov, D. Olson, L. G. de Lima, T. Potapova, G. A. Hartley, M. Haukness, P. Kerpedjiev, F. Gusev, K. Tigyi, S. Brooks, A. Young, S. Nurk, S. Koren, S. R. Salama, B. Paten, E. I. Rogaev, A. Streets, G. H. Karpen, A. F. Dernburg, B. A. Sullivan, A. F. Straight, T. J. Wheeler, J. L. Gerton, E. E. Eichler, A. M. Phillippy, W. Timp, Complete genomic and epigenetic maps of human centromeres. Science 376, eabl4178 (2022). - PMC - PubMed
-
- G. A. Logsdon, M. R. Vollger, P. Hsieh, Y. Mao, M. A. Liskovykh, S. Koren, S. Nurk, L. Mercuri, P. C. Dishuck, A. Rhie, L. G. de Lima, T. Dvorkina, D. Porubsky, W. T. Harvey, A. Mikheenko, A. V. Bzikadze, M. Kremitzki, T. A. Graves-Lindsay, C. Jain, K. Hoekzema, S. C. Murali, K. M. Munson, C. Baker, M. Sorensen, A. M. Lewis, U. Surti, J. L. Gerton, V. Larionov, M. Ventura, K. H. Miga, A. M. Phillippy, E. E. Eichler, The structure, function and evolution of a complete human chromosome 8. Nature 593, 101–107 (2021). - PMC - PubMed
-
- K. H. Miga, S. Koren, A. Rhie, M. R. Vollger, A. Gershman, A. Bzikadze, S. Brooks, E. Howe, D. Porubsky, G. A. Logsdon, V. A. Schneider, T. Potapova, J. Wood, W. Chow, J. Armstrong, J. Fredrickson, E. Pak, K. Tigyi, M. Kremitzki, C. Markovic, V. Maduro, A. Dutra, G. G. Bouffard, A. M. Chang, N. F. Hansen, A. B. Wilfert, F. Thibaud-Nissen, A. D. Schmitt, J.-M. Belton, S. Selvaraj, M. Y. Dennis, D. C. Soto, R. Sahasrabudhe, G. Kaya, J. Quick, N. J. Loman, N. Holmes, M. Loose, U. Surti, R. A. Risques, T. A. Graves Lindsay, R. Fulton, I. Hall, B. Paten, K. Howe, W. Timp, A. Young, J. C. Mullikin, P. A. Pevzner, J. L. Gerton, B. A. Sullivan, E. E. Eichler, A. M. Phillippy, Telomere-to-telomere assembly of a complete human X chromosome. Nature 585, 79–84 (2020). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

