Operando Electron Microscopy of Catalysts: The Missing Cornerstone in Heterogeneous Catalysis Research?
- PMID: 37967448
- PMCID: PMC10722467
- DOI: 10.1021/acs.chemrev.3c00352
Operando Electron Microscopy of Catalysts: The Missing Cornerstone in Heterogeneous Catalysis Research?
Abstract
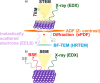
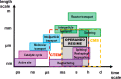
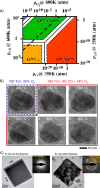
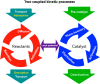
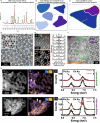
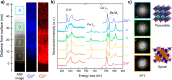
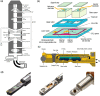
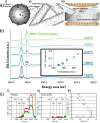
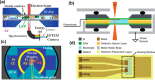
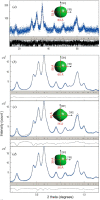
Heterogeneous catalysis in thermal gas-phase and electrochemical liquid-phase chemical conversion plays an important role in our modern energy landscape. However, many of the structural features that drive efficient chemical energy conversion are still unknown. These features are, in general, highly distinct on the local scale and lack translational symmetry, and thus, they are difficult to capture without the required spatial and temporal resolution. Correlating these structures to their function will, conversely, allow us to disentangle irrelevant and relevant features, explore the entanglement of different local structures, and provide us with the necessary understanding to tailor novel catalyst systems with improved productivity. This critical review provides a summary of the still immature field of operando electron microscopy for thermal gas-phase and electrochemical liquid-phase reactions. It focuses on the complexity of investigating catalytic reactions and catalysts, progress in the field, and analysis. The forthcoming advances are discussed in view of correlative techniques, artificial intelligence in analysis, and novel reactor designs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures













References
-
- Schmal M.Heterogeneous Catalysis and Its Industrial Applications; Springer, 2016.
-
- Hagen J.Economic Importance of Catalysts. In Industrial Catalysis; Wiley, 2015; pp 459–462.
-
- Chorkendorff I.; Niemantsverdriet J. W.. Concepts of Modern Catalysis and Kinetics, 3rd ed.; Wiley-VCH Verlag, 2003.
-
- Olah G. A.; Goeppert A.; Prakash G. K. S.. Beyond Oil and Gas: The Methanol Economy, 2nd ed.; Wiley, 2009.
-
- Chemical Energy Storage; Schlögl R., Ed.; De Gruyter, 2013.
Publication types
LinkOut - more resources
Full Text Sources

