Antibacterial Synthetic Nanocelluloses Synergizing with a Metal-Chelating Agent
- PMID: 37967519
- PMCID: PMC10792664
- DOI: 10.1021/acsabm.3c00846
Antibacterial Synthetic Nanocelluloses Synergizing with a Metal-Chelating Agent
Abstract
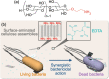
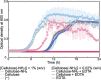
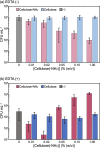
Antibacterial materials composed of biodegradable and biocompatible constituents that are produced via eco-friendly synthetic strategies will become an attractive alternative to antibiotics to combat antibiotic-resistant bacteria. In this study, we demonstrated the antibacterial properties of nanosheet-shaped crystalline assemblies of enzymatically synthesized aminated cellulose oligomers (namely, surface-aminated synthetic nanocelluloses) and their synergy with a metal-chelating antibacterial agent, ethylenediaminetetraacetic acid (EDTA). Growth curves and colony counting assays revealed that the surface-aminated cellulose assemblies had an antibacterial effect against Gram-negative Escherichia coli (E. coli). The cationic assemblies appeared to destabilize the cell wall of E. coli through electrostatic interactions with anionic lipopolysaccharide (LPS) molecules on the outer membrane. The antibacterial properties were significantly enhanced by the concurrent use of EDTA, which potentially removed metal ions from LPS molecules, resulting in synergistic bactericidal effects. No antibacterial activity of the surface-aminated cellulose assemblies was observed against Gram-positive Staphylococcus aureus even in the presence of EDTA, further supporting the contribution of electrostatic interactions between the cationic assemblies and anionic LPS to the activity against Gram-negative bacteria. Analysis using quartz crystal microbalance with dissipation monitoring revealed the attractive interaction of the surface-aminated cellulose assembly with LPS Ra monolayers artificially produced on the device substrate.
Keywords: antibacterial cationic polymer; bactericidal activity; cellulose oligomer; crystalline assembly; ethylenediaminetetraacetic acid; synergistic effect.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Ionic interaction-driven switchable bactericidal surfaces.Acta Biomater. 2022 Apr 1;142:124-135. doi: 10.1016/j.actbio.2022.02.003. Epub 2022 Feb 9. Acta Biomater. 2022. PMID: 35149242
-
Synergistic Actions of Benzyl Isothiocyanate with Ethylenediaminetetraacetic Acid and Efflux Pump Inhibitor Phenylalanine-Arginine β-Naphthylamide Against Multidrug-Resistant Escherichia coli.Microb Drug Resist. 2020 May;26(5):468-474. doi: 10.1089/mdr.2019.0118. Epub 2019 Nov 22. Microb Drug Resist. 2020. PMID: 31755808
-
Studies on the antibacterial activity of phanquone: chelating properties in relation to mode of action against Escherichia coli and Staphylococcus aureus.Microbios. 1980;29(116):109-25. Microbios. 1980. PMID: 7022141
-
Active insolubilized antibiotics based on cellulose-metal chelates.Antimicrob Agents Chemother. 1974 Dec;6(6):777-82. doi: 10.1128/AAC.6.6.777. Antimicrob Agents Chemother. 1974. PMID: 4451349 Free PMC article.
-
Effect of certain chelating agents on the antibacterial action of silver nitrate.J Hyg Epidemiol Microbiol Immunol. 1988;32(3):299-306. J Hyg Epidemiol Microbiol Immunol. 1988. PMID: 3143759
Cited by
-
Surface-mediated self-assembly of click-reactive cello-oligosaccharides for fabricating functional nonwoven fabrics.Sci Technol Adv Mater. 2024 Feb 6;25(1):2311052. doi: 10.1080/14686996.2024.2311052. eCollection 2024. Sci Technol Adv Mater. 2024. PMID: 38361530 Free PMC article.
-
Borosilicate bioactive glass synergizing low-dose antibiotic loaded implants to combat bacteria through ATP disruption and oxidative stress to sequentially achieve osseointegration.Bioact Mater. 2024 Oct 18;44:184-204. doi: 10.1016/j.bioactmat.2024.10.009. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39502840 Free PMC article.
References
-
- Ding X.; Duan S.; Ding X.; Liu R.; Xu F.-J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1802140.10.1002/adfm.201802140. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
