Fiber-deficient diet inhibits colitis through the regulation of the niche and metabolism of a gut pathobiont
- PMID: 37967555
- PMCID: PMC10842462
- DOI: 10.1016/j.chom.2023.10.016
Fiber-deficient diet inhibits colitis through the regulation of the niche and metabolism of a gut pathobiont
Abstract
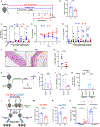
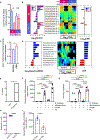
Exclusive enteral nutrition (EEN) with fiber-free diets is an effective steroid-sparing treatment to induce clinical remission in children with Crohn's disease (CD). However, the mechanism underlying the beneficial effects of EEN remains obscure. Using a model of microbiota-dependent colitis with the hallmarks of CD, we find that the administration of a fiber-free diet prevents the development of colitis and inhibits intestinal inflammation in colitic animals. Remarkably, fiber-free diet alters the intestinal localization of Mucispirillum schaedleri, a mucus-dwelling pathobiont, which is required for triggering disease. Mechanistically, the absence of dietary fiber reduces nutrient availability and impairs the dissimilatory nitrate reduction to ammonia (DNRA) metabolic pathway of Mucispirillum, leading to its exclusion from the mucus layer and disease remission. Thus, appropriate localization of the specific pathobiont in the mucus layer is critical for disease development, which is disrupted by fiber exclusion. These results suggest strategies to treat CD by targeting the intestinal niche and metabolism of disease-causing microbes.
Keywords: Crohn’s disease; Mucispirillum schaedleri; Ruminococcus torques; dissimilatory nitrate reduction to ammonia; exclusive enteral nutrition; fiber-free diet; mucus-dwelling pathobiont.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Fiber-deficient diets reprogram the microbiota.Cell Host Microbe. 2023 Dec 13;31(12):1950-1951. doi: 10.1016/j.chom.2023.11.014. Cell Host Microbe. 2023. PMID: 38096786
References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

