Proteomics from compartment-specific APEX2 labeling in Mycobacterium tuberculosis reveals Type VII secretion substrates in the cell wall
- PMID: 37967559
- PMCID: PMC11106752
- DOI: 10.1016/j.chembiol.2023.10.013
Proteomics from compartment-specific APEX2 labeling in Mycobacterium tuberculosis reveals Type VII secretion substrates in the cell wall
Abstract
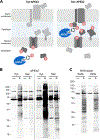
The cell wall of mycobacteria plays a key role in interactions with the environment. Its ability to act as a selective filter is crucial to bacterial survival. Proteins in the cell wall enable this function by mediating the import and export of diverse metabolites, from ions to lipids to proteins. Identifying cell wall proteins is an important step in assigning function, especially as many mycobacterial proteins lack functionally characterized homologues. Current methods for protein localization have inherent limitations that reduce accuracy. Here we showed that although chemical labeling of live cells did not exclusively label surface proteins, protein tagging by the engineered peroxidase APEX2 within live Mycobacterium tuberculosis accurately identified the cytosolic and cell wall proteomes. Our data indicate that substrates of the virulence-associated Type VII ESX secretion system are exposed to the periplasm, providing insight into the currently unknown mechanism by which these proteins cross the mycobacterial cell envelope.
Keywords: APEX2; ESX; Type VII secretion; cell wall; mycobacteria; periplasm; proximity labeling; tuberculosis.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
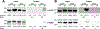

Similar articles
-
Cell wall proteomics in live Mycobacterium tuberculosis uncovers exposure of ESX substrates to the periplasm.bioRxiv [Preprint]. 2023 Mar 29:2023.03.29.534792. doi: 10.1101/2023.03.29.534792. bioRxiv. 2023. PMID: 37034674 Free PMC article. Preprint.
-
Type VII Secretion Substrates of Pathogenic Mycobacteria Are Processed by a Surface Protease.mBio. 2019 Oct 29;10(5):e01951-19. doi: 10.1128/mBio.01951-19. mBio. 2019. PMID: 31662454 Free PMC article.
-
The ESX-1 Substrate PPE68 Has a Key Function in ESX-1-Mediated Secretion in Mycobacterium marinum.mBio. 2022 Dec 20;13(6):e0281922. doi: 10.1128/mbio.02819-22. Epub 2022 Nov 21. mBio. 2022. PMID: 36409073 Free PMC article.
-
Structure and Function of the Mycobacterial Type VII Secretion Systems.Annu Rev Microbiol. 2020 Sep 8;74:315-335. doi: 10.1146/annurev-micro-012420-081657. Epub 2020 Jul 13. Annu Rev Microbiol. 2020. PMID: 32660388 Review.
-
ESX/Type VII Secretion Systems-An Important Way Out for Mycobacterial Proteins.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.psib-0029-2019. doi: 10.1128/microbiolspec.PSIB-0029-2019. Microbiol Spectr. 2019. PMID: 31298207 Free PMC article. Review.
Cited by
-
Metabolic tagging reveals surface-associated lipoproteins in mycobacteria.bioRxiv [Preprint]. 2025 May 5:2025.01.07.631728. doi: 10.1101/2025.01.07.631728. bioRxiv. 2025. PMID: 39829771 Free PMC article. Preprint.
-
A split ALFA tag-nanobody system for protein localization and proximity proteomics in mycobacteria.bioRxiv [Preprint]. 2025 Mar 28:2025.03.22.644702. doi: 10.1101/2025.03.22.644702. bioRxiv. 2025. Update in: mBio. 2025 Aug 13;16(8):e0097125. doi: 10.1128/mbio.00971-25. PMID: 40196699 Free PMC article. Updated. Preprint.
-
A split ALFA tag-nanobody system for protein localization and proximity proteomics in mycobacteria.mBio. 2025 Aug 13;16(8):e0097125. doi: 10.1128/mbio.00971-25. Epub 2025 Jun 27. mBio. 2025. PMID: 40576343 Free PMC article.
-
Protease shaving of Mycobacterium tuberculosis facilitates vaccine antigen discovery and delivery of novel cargoes to the Mtb surface.Microbiol Spectr. 2025 Feb 4;13(2):e0227724. doi: 10.1128/spectrum.02277-24. Epub 2024 Dec 17. Microbiol Spectr. 2025. PMID: 39688428 Free PMC article.
-
A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope.Microorganisms. 2024 Sep 4;12(9):1827. doi: 10.3390/microorganisms12091827. Microorganisms. 2024. PMID: 39338501 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

