Computational prediction of complex cationic rearrangement outcomes
- PMID: 37967579
- PMCID: PMC10864989
- DOI: 10.1038/s41586-023-06854-3
Computational prediction of complex cationic rearrangement outcomes
Abstract
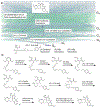
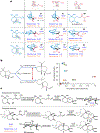
Recent years have seen revived interest in computer-assisted organic synthesis1,2. The use of reaction- and neural-network algorithms that can plan multistep synthetic pathways have revolutionized this field1,3-7, including examples leading to advanced natural products6,7. Such methods typically operate on full, literature-derived 'substrate(s)-to-product' reaction rules and cannot be easily extended to the analysis of reaction mechanisms. Here we show that computers equipped with a comprehensive knowledge-base of mechanistic steps augmented by physical-organic chemistry rules, as well as quantum mechanical and kinetic calculations, can use a reaction-network approach to analyse the mechanisms of some of the most complex organic transformations: namely, cationic rearrangements. Such rearrangements are a cornerstone of organic chemistry textbooks and entail notable changes in the molecule's carbon skeleton8-12. The algorithm we describe and deploy at https://HopCat.allchemy.net/ generates, within minutes, networks of possible mechanistic steps, traces plausible step sequences and calculates expected product distributions. We validate this algorithm by three sets of experiments whose analysis would probably prove challenging even to highly trained chemists: (1) predicting the outcomes of tail-to-head terpene (THT) cyclizations in which substantially different outcomes are encoded in modular precursors differing in minute structural details; (2) comparing the outcome of THT cyclizations in solution or in a supramolecular capsule; and (3) analysing complex reaction mixtures. Our results support a vision in which computers no longer just manipulate known reaction types1-7 but will help rationalize and discover new, mechanistically complex transformations.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures



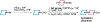
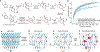



Similar articles
-
Computational planning of the synthesis of complex natural products.Nature. 2020 Dec;588(7836):83-88. doi: 10.1038/s41586-020-2855-y. Epub 2020 Oct 13. Nature. 2020. PMID: 33049755
-
Syntheses of Complex Terpenes from Simple Polyprenyl Precursors.Acc Chem Res. 2020 Apr 21;53(4):949-961. doi: 10.1021/acs.accounts.0c00055. Epub 2020 Mar 23. Acc Chem Res. 2020. PMID: 32202757 Free PMC article. Review.
-
Total synthesis of complex terpenoids employing radical cascade processes.Nat Prod Rep. 2018 Feb 21;35(2):174-202. doi: 10.1039/c7np00065k. Nat Prod Rep. 2018. PMID: 29417970 Free PMC article. Review.
-
Terpene cyclization catalysed inside a self-assembled cavity.Nat Chem. 2015 Mar;7(3):197-202. doi: 10.1038/nchem.2181. Epub 2015 Feb 16. Nat Chem. 2015. PMID: 25698327
-
Synthesis of highly strained terpenes by non-stop tail-to-head polycyclization.Nat Chem. 2012 Nov;4(11):915-20. doi: 10.1038/nchem.1458. Epub 2012 Sep 23. Nat Chem. 2012. PMID: 23089866
Cited by
-
Retro-forward synthesis design and experimental validation of potent structural analogs of known drugs.Chem Sci. 2025 Mar 19;16(19):8383-8393. doi: 10.1039/d5sc00070j. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40225181 Free PMC article.
-
Expanding Meroterpenoid Chemical Space Via Intermolecular Trapping of Cationic Cyclization Intermediates.JACS Au. 2025 Jun 27;5(7):3021-3026. doi: 10.1021/jacsau.5c00492. eCollection 2025 Jul 28. JACS Au. 2025. PMID: 40747079 Free PMC article.
-
Computer-Generated, Mechanistic Networks Assist in Assigning the Outcomes of Complex Multicomponent Reactions.J Am Chem Soc. 2025 May 7;147(18):15636-15644. doi: 10.1021/jacs.5c02846. Epub 2025 Apr 28. J Am Chem Soc. 2025. PMID: 40293375 Free PMC article.
References
-
- Szymkuć S et al. Computer-assisted synthetic planning: the end of the beginning. Angew. Chem. Int. Ed 55, 5904–5937 (2016). - PubMed
-
- Corey EJ & Wipke WT Computer-assisted design of complex organic syntheses. Science 166, 178–192 (1969). - PubMed
-
- Klucznik T et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4, 522–532 (2018).
-
- Segler MHS, Preuss M & Waller MP Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018). - PubMed
-
- Coley CW, Green WH & Jensen KF Machine learning in computer-aided synthesis planning. Acc. Chem. Res 51, 1281–1289 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

