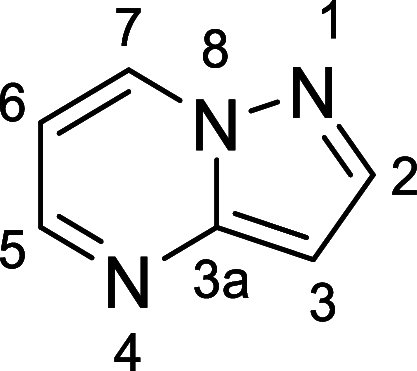
Discovery of KB-0742, a Potent, Selective, Orally Bioavailable Small Molecule Inhibitor of CDK9 for MYC-Dependent Cancers
- PMID: 37967851
- PMCID: PMC10726352
- DOI: 10.1021/acs.jmedchem.3c01233
Discovery of KB-0742, a Potent, Selective, Orally Bioavailable Small Molecule Inhibitor of CDK9 for MYC-Dependent Cancers
Abstract
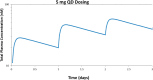
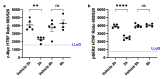
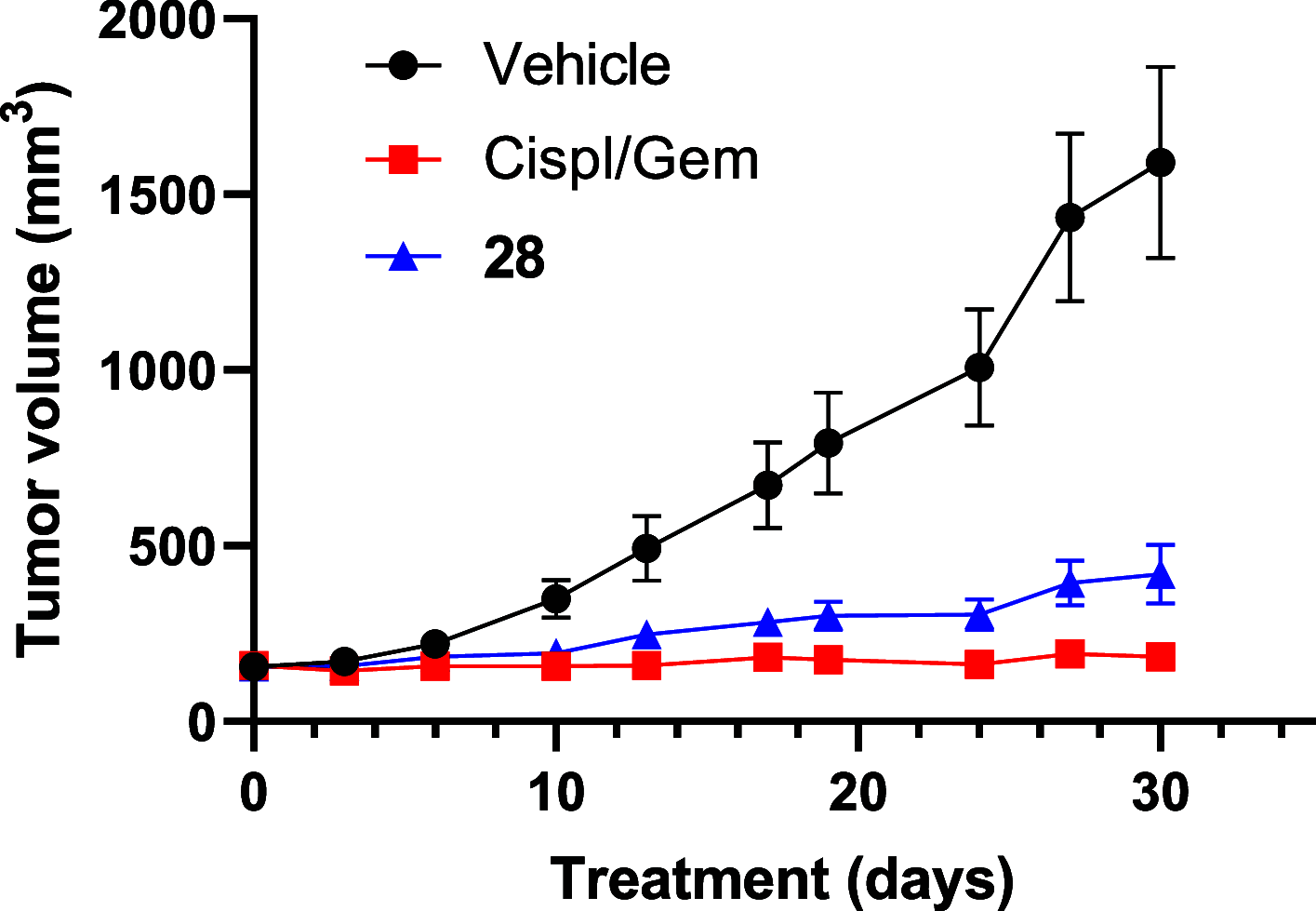
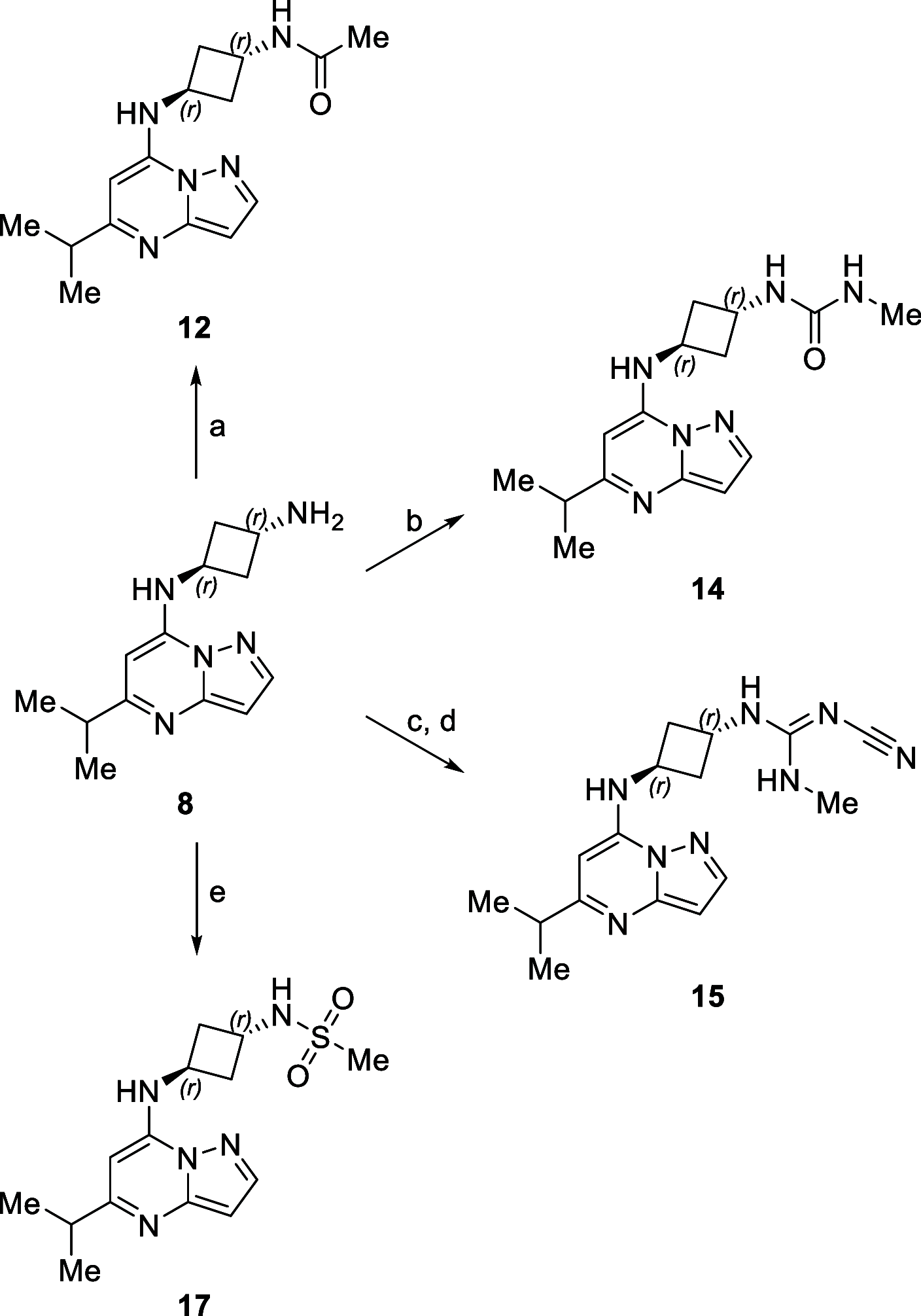
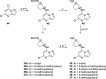
Transcriptional deregulation is a hallmark of many cancers and is exemplified by genomic amplifications of the MYC family of oncogenes, which occur in at least 20% of all solid tumors in adults. Targeting of transcriptional cofactors and the transcriptional cyclin-dependent kinase (CDK9) has emerged as a therapeutic strategy to interdict deregulated transcriptional activity including oncogenic MYC. Here, we report the structural optimization of a small molecule microarray hit, prioritizing maintenance of CDK9 selectivity while improving on-target potency and overall physicochemical and pharmacokinetic (PK) properties. This led to the discovery of the potent, selective, orally bioavailable CDK9 inhibitor 28 (KB-0742). Compound 28 exhibits in vivo antitumor activity in mouse xenograft models and a projected human PK profile anticipated to enable efficacious oral dosing. Notably, 28 is currently being investigated in a phase 1/2 dose escalation and expansion clinical trial in patients with relapsed or refractory solid tumors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

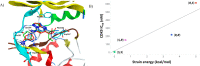


References
-
- Schaub F. X.; Dhankani V.; Berger A. C.; Trivedi M.; Richardson A. B.; Shaw R.; Zhao W.; Zhang X.; Ventura A.; Liu Y.; Ayer D. E.; Hurlin P. J.; Cherniack A. D.; Eisenman R. N.; Bernard B.; Grandori C.; et al. Pan-Cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst. 2018, 6 (3), 282–300.e2. 10.1016/j.cels.2018.03.003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Miscellaneous

