Real-World Treatment Patterns and Clinical Outcomes in Korean Patients With AML Ineligible for First-Line Intensive Chemotherapy: A Subanalysis of the CURRENT Study, a Non-Interventional, Retrospective Chart Review
- PMID: 37967874
- PMCID: PMC10643247
- DOI: 10.3346/jkms.2023.38.e345
Real-World Treatment Patterns and Clinical Outcomes in Korean Patients With AML Ineligible for First-Line Intensive Chemotherapy: A Subanalysis of the CURRENT Study, a Non-Interventional, Retrospective Chart Review
Abstract
Background: Although most elderly patients with acute myeloid leukemia (AML) are ineligible for intensive chemotherapy (ICT), treatment options remain limited. CURRENT (UMIN000037786), a real-world, non-interventional, retrospective chart review, evaluated clinical outcomes, clinicopathologic characteristics, and treatment patterns in these patients. We present results from a subanalysis of Korean patients in this study.
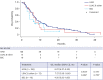
Methods: Patients were aged ≥ 18 years with primary or secondary AML ineligible for ICT who initiated first-line systemic therapy or best supportive care (BSC) between 2015 and 2018 across four centers in Korea. Primary endpoint was overall survival (OS) from diagnosis. Secondary endpoints included progression-free survival (PFS), time to treatment failure, and response rates. Data analyses were primarily descriptive, with time-to-event outcomes estimated using the Kaplan-Meier method, and Cox regression used to determine prognostic factors for survival.
Results: Among 194 patients enrolled, 84.0% received systemic therapy and 16.0% received BSC. Median age at diagnosis was 74 and 78 years, and Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1 was reported in 73.0% and 48.4% of patients, respectively; poor cytogenetic risk was reported in 30.1% and 16.1% of patients. Median OS was 7.83 vs. 4.50 months, and median PFS was 6.73 vs. 4.50 months in the systemic therapy vs. BSC groups. Prognostic factors affecting OS included secondary AML (hazard ratio, 1.67 [95% confidence interval, 1.13-2.45]), ECOG performance status ≥ 2 (2.41 [1.51-3.83]), poor cytogenetic risk (2.10 [1.36-3.24]), and Charlson comorbidity index ≥ 1 (2.26 [1.43-3.58]).
Conclusion: Clinical outcomes are poor in Korean patients with AML ineligible for ICT who are prescribed current systemic therapies or BSC. There is a substantial unmet need for novel agents (monotherapy or in combination) to improve clinical outcomes in this patient population.
Keywords: Acute Myeloid Leukemia; Clinical Outcomes; Korea; Real-World; Treatment Patterns.
© 2023 The Korean Academy of Medical Sciences.
Conflict of interest statement
AbbVie had the following involvement with the study: funding for study design, data collection, analysis, interpretation, and medical writing for the manuscript. Llamas C, Duan Y and Jeong JY are employees of AbbVie. Llamas C and Duan Y were involved in the study conceptualization, data curation, and the reviewing and editing of the manuscript. Jeong JY was involved in, formal analysis, and the writing, reviewing, and editing of the manuscript. Lee JH had advisory roles for AbbVie, Astellas, Celgene, Janssen, and Novartis. All other authors (Bang SM, Kang KW, and Song IC) declare no competing interests. Llamas C, Duan Y and Jeong JY are employees of AbbVie, and Lee JH had advisory roles for AbbVie, Astellas, Celgene, Janssen, and Novartis. All other authors (Bang SM, Kang KW, and Song IC) declare no competing interests.
Figures
Similar articles
-
Real-world treatment patterns and clinical outcomes in patients with AML in Japan who were ineligible for first-line intensive chemotherapy.Int J Hematol. 2022 Jul;116(1):89-101. doi: 10.1007/s12185-022-03334-8. Epub 2022 Apr 8. Int J Hematol. 2022. PMID: 35394258
-
Real-world treatment patterns and clinical outcomes in patients with AML unfit for first-line intensive chemotherapy.Leuk Lymphoma. 2022 Apr;63(4):928-938. doi: 10.1080/10428194.2021.2002321. Epub 2022 Feb 11. Leuk Lymphoma. 2022. PMID: 35147482
-
Healthcare resource utilization trends in patients with acute myeloid leukemia ineligible for intensive chemotherapy receiving first-line systemic treatment or best supportive care: A multicenter international study.Eur J Haematol. 2022 Jul;109(1):58-68. doi: 10.1111/ejh.13769. Epub 2022 Apr 13. Eur J Haematol. 2022. PMID: 35298049 Free PMC article.
-
Venetoclax for the treatment of elderly or chemotherapy-ineligible patients with acute myeloid leukemia: a step in the right direction or a game changer?Expert Rev Hematol. 2021 Feb;14(2):199-210. doi: 10.1080/17474086.2021.1876559. Epub 2021 Feb 8. Expert Rev Hematol. 2021. PMID: 33459064 Review.
-
Conventional chemotherapy or hypomethylating agents for older patients with acute myeloid leukaemia?Hematol Oncol. 2014 Mar;32(1):1-9. doi: 10.1002/hon.2046. Epub 2013 Mar 20. Hematol Oncol. 2014. PMID: 23512815 Review.
References
-
- Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, et al. NCCN guidelines insights: acute myeloid leukemia, version 2. 2021. J Natl Compr Canc Netw. 2021;19(1):16–27. - PubMed
-
- National Cancer Institute. Cancer stat facts: leukemia — acute myeloid leukemia. [Updated 2021]. [Accessed September 12, 2023]. https://seer.cancer.gov/statfacts/html/amyl.html .
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

