BRISC is required for optimal activation of NF-κB in Kupffer cells induced by LPS and contributes to acute liver injury
- PMID: 37968261
- PMCID: PMC10651896
- DOI: 10.1038/s41419-023-06268-z
BRISC is required for optimal activation of NF-κB in Kupffer cells induced by LPS and contributes to acute liver injury
Abstract
BRISC (BRCC3 isopeptidase complex) is a deubiquitinating enzyme that has been linked with inflammatory processes, but its role in liver diseases and the underlying mechanism are unknown. Here, we investigated the pathophysiological role of BRISC in acute liver failure using a mice model induced by D-galactosamine (D-GalN) plus lipopolysaccharide (LPS). We found that the expression of BRISC components was dramatically increased in kupffer cells (KCs) upon LPS treatment in vitro or by the injection of LPS in D-GalN-sensitized mice. D-GalN plus LPS-induced liver damage and mortality in global BRISC-null mice were markedly attenuated, which was accompanied by impaired hepatocyte death and hepatic inflammation response. Constantly, treatment with thiolutin, a potent BRISC inhibitor, remarkably alleviated D-GalN/LPS-induced liver injury in mice. By using bone marrow-reconstituted chimeric mice and cell-specific BRISC-deficient mice, we demonstrated that KCs are the key effector cells responsible for protection against D-GalN/LPS-induced liver injury in BRISC-deficient mice. Mechanistically, we found that hepatic and circulating levels of TNF-α, IL-6, MCP-1, and IL-1β, as well as TNF-α- and MCP-1-producing KCs, in BRISC-deleted mice were dramatically decreased as early as 1 h after D-GalN/LPS challenge, which occurred prior to the elevation of the liver injury markers. Moreover, LPS-induced proinflammatory cytokines production in KCs was significantly diminished by BRISC deficiency in vitro, which was accompanied by potently attenuated NF-κB activation. Restoration of NF-κB activation by two small molecular activators of NF-κB p65 effectively reversed the suppression of cytokines production in ABRO1-deficient KCs by LPS. In conclusion, BRISC is required for optimal activation of NF-κB-mediated proinflammatory cytokines production in LPS-treated KCs and contributes to acute liver injury. This study opens the possibility to develop new strategies for the inhibition of KCs-driven inflammation in liver diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

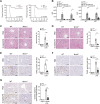


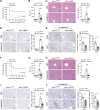



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

