Pathway selection in the self-assembly of Rh4L4 coordination squares under kinetic control
- PMID: 37968322
- PMCID: PMC10651846
- DOI: 10.1038/s42004-023-01053-7
Pathway selection in the self-assembly of Rh4L4 coordination squares under kinetic control
Abstract
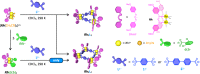
Pathway selection principles in reversible reaction networks such as molecular self-assembly have not been established yet, because achieving kinetic control in reversible reaction networks is more complicated than in irreversible ones. In this study, we discovered that coordination squares consisting of cis-protected dinuclear rhodium(II) corner complexes and linear ditopic ligands are assembled under kinetic control, perfectly preventing the corresponding triangles, by modulating their energy landscapes with a weak monotopic carboxylate ligand (2,6-dichlorobenzoate: dcb-) as the leaving ligand. Experimental and numerical approaches revealed the self-assembly pathway where the cyclization step to form the triangular complex is blocked by dcb-. It was also found that one of the molecular squares assembled into a dimeric structure owing to the solvophobic effect, which was characterized by nuclear magnetic resonance spectroscopy and single-crystal X-ray analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dimmock, N. J., Easton, A. J. & Leppard, K. N. Introduction to Modern Virology, 7th Ed. Wiley-Blackwell, (2016).
-
- Takahashi S, Abe T, Sato H, Hiraoka S. Pathway bias and emergence of quasi-irreversibility in reversible reaction networks: Extension of Curtin-Hammett principle. Chem. 2023;9:2971–2982. doi: 10.1016/j.chempr.2023.06.015. - DOI
-
- Fujita, M., Yazaki, J. & Ogura, K. Preparation of a macrocyclic polynuclear complex, [(en)Pd(4,4′-bpy)]4(NO3)8 (en = ethylenediamine, bpy = bipyridine), which recognizes an organic molecule in aqueous media. J. Am. Chem. Soc.112, 5645–5647 (1990).
Grants and funding
- 19H02731/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23H01970/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19K22196/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K18974/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K04663/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources

