iPSC-derived microglia carrying the TREM2 R47H/+ mutation are proinflammatory and promote synapse loss
- PMID: 37969043
- PMCID: PMC10904109
- DOI: 10.1002/glia.24485
iPSC-derived microglia carrying the TREM2 R47H/+ mutation are proinflammatory and promote synapse loss
Abstract
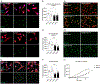
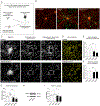
Genetic findings have highlighted key roles for microglia in the pathology of neurodegenerative conditions such as Alzheimer's disease (AD). A number of mutations in the microglial protein triggering receptor expressed on myeloid cells 2 (TREM2) have been associated with increased risk for developing AD, most notably the R47H/+ substitution. We employed gene editing and stem cell models to gain insight into the effects of the TREM2 R47H/+ mutation on human-induced pluripotent stem cell-derived microglia. We found transcriptional changes affecting numerous cellular processes, with R47H/+ cells exhibiting a proinflammatory gene expression signature. TREM2 R47H/+ also caused impairments in microglial movement and the uptake of multiple substrates, as well as rendering microglia hyperresponsive to inflammatory stimuli. We developed an in vitro laser-induced injury model in neuron-microglia cocultures, finding an impaired injury response by TREM2 R47H/+ microglia. Furthermore, mouse brains transplanted with TREM2 R47H/+ microglia exhibited reduced synaptic density, with upregulation of multiple complement cascade components in TREM2 R47H/+ microglia suggesting inappropriate synaptic pruning as one potential mechanism. These findings identify a number of potentially detrimental effects of the TREM2 R47H/+ mutation on microglial gene expression and function likely to underlie its association with AD.
Keywords: Alzheimer's disease; induced pluripotent stem cells; inflammation; microglia; neurodegeneration; triggering receptor expressed on myeloid cells 2 (TREM2).
© 2023 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
Figures



References
-
- Andreone BJ, Przybyla L, Llapashtica C, Rana A, Davis SS, van Lengerich B, Lin K, Shi J, Mei Y, Astarita G, Di Paolo G, Sandmann T, Monroe KM, Lewcock JW. 2020. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat Neurosci 23:927–938. doi:10.1038/s41593-020-0650-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

