Bone marrow stromal cell antigen-1 deficiency protects from acute kidney injury
- PMID: 37969103
- PMCID: PMC11967511
- DOI: 10.1152/ajprenal.00175.2023
Bone marrow stromal cell antigen-1 deficiency protects from acute kidney injury
Abstract
This study aimed to investigate the role of bone marrow stromal cell antigen-1 (Bst1; also known as CD157) in acute kidney injury (AKI). Bst1 is a cell surface molecule with various enzymatic activities and downstream intracellular signaling pathways that modulate the immune response. Previous research has linked Bst1 to diseases such as ovarian cancer, Parkinson's disease, and rheumatoid arthritis. We used bilateral ischemia-reperfusion injury (IRI) as an AKI model and created bone marrow chimeric mice to evaluate the role of Bst1 in bone marrow-derived cells. We also used flow cytometry to identify Bst1/CD157 expression in hematopoietic cells and evaluate immune cell dynamics in the kidney. The findings showed that Bst1-deficient (Bst1-/-) mice were protected against renal bilateral IRI. Bone marrow chimera experiments revealed that Bst1 expression on hematopoietic cells, but not parenchymal cells, induced renal IRI. Bst1 was mainly found in B cells and neutrophils by flow cytometry of the spleen and bone marrow. In vitro, migration of neutrophils from Bst1-/- mice was suppressed, and adoptive transfer of neutrophils from wild-type Bst1+/+ mice abolished the renal protective effect in Bst1 knockout mice. In conclusion, the study demonstrated that Bst1-/- mice are protected against renal IRI and that Bst1 expression in neutrophils plays a crucial role in inducing renal IRI. These findings suggest that targeting Bst1 in neutrophils could be a potential therapeutic strategy for AKI.NEW & NOTEWORTHY Acute kidney injury (AKI), a serious disease for which there is no effective Federal Drug Administration-approved treatment, is associated with high mortality rates. Bone marrow stromal cell antigen-1 (Bst1) is a cell surface molecule that can cause kidney fibrosis, but its role in AKI is largely unknown. Our study showed that Bst1-/- mice revealed a protective effect against renal bilateral ischemia-reperfusion injury (IRI). Adoptive transfer studies confirmed that Bst1 expression in hematopoietic cells, especially neutrophils, contributed to renal bilateral IRI.
Keywords: CD157; acute kidney injury; bone marrow stromal cell antigen-1; neutrophils.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

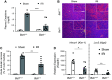

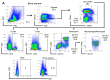

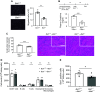
Comment in
-
Beast of (renal) burden? Bst1-expressing neutrophils in kidney injury.Am J Physiol Renal Physiol. 2024 Feb 1;326(2):F165-F166. doi: 10.1152/ajprenal.00386.2023. Epub 2023 Dec 14. Am J Physiol Renal Physiol. 2024. PMID: 38095024 No abstract available.
References
-
- Liu KD, Goldstein SL, Vijayan A, Parikh CR, Kashani K, Okusa MD, Agarwal A, Cerdá J; AKI!Now Initiative of the American Society of Nephrology. AKI!Now initiative: recommendations for awareness, recognition, and management of AKI. Clin J Am Soc Nephrol 15: 1838–1847, 2020. doi:10.2215/CJN.15611219. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01DK062324/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- JP22gm6210013/Japan Agency for Medical Research and Development (AMED)
- R01 DK085259/DK/NIDDK NIH HHS/United States
- 20K17242/MEXT | Japan Society for the Promotion of Science (JSPS)
- 1S10RR026799-01/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- 22H03090/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01 DK062324/DK/NIDDK NIH HHS/United States
- R01 AI148231/AI/NIAID NIH HHS/United States
- 18H06192/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJFR210J/MEXT | JST | Fusion Oriented REsearch for disruptive Science and Technology (FOREST)
- S10 RR026799/RR/NCRR NIH HHS/United States
- R01AI148231/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01DK085259/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

