Does Heat Play a Role in the Observed Behavior of Aqueous Photobatteries?
- PMID: 37969251
- PMCID: PMC10644369
- DOI: 10.1021/acsenergylett.3c01627
Does Heat Play a Role in the Observed Behavior of Aqueous Photobatteries?
Abstract
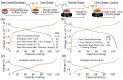
Light-rechargeable photobatteries have emerged as an elegant solution to address the intermittency of solar irradiation by harvesting and storing solar energy directly through a battery electrode. Recently, a number of compact two-electrode photobatteries have been proposed, showing increases in capacity and open-circuit voltage upon illumination. Here, we analyze the thermal contributions to this increase in capacity under galvanostatic and photocharging conditions in two promising photoactive cathode materials, V2O5 and LiMn2O4. We propose an improved cell and experimental design and perform temperature-controlled photoelectrochemical measurements using these materials as photocathodes. We show that the photoenhanced capacities of these materials under 1 sun irradiation can be attributed mostly to thermal effects. Using operando reflection spectroscopy, we show that the spectral behavior of the photocathode changes as a function of the state of charge, resulting in changing optical absorption properties. Through this technique, we show that the band gap of V2O5 vanishes after continued zinc ion intercalation, making it unsuitable as a photocathode beyond a certain discharge voltage. These results and experimental techniques will enable the rational selection and testing of materials for next-generation photo-rechargeable systems.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Kannan N.; Vakeesan D. Solar energy for future world:-a review. Renew. Sust. Energy Rev. 2016, 62, 1092–1105. 10.1016/j.rser.2016.05.022. - DOI
-
- Hou Q.; Zhang N.; Du E.; Miao M.; Peng F.; Kang C. Probabilistic duck curve in high PV penetration power system: Concept, modeling, and empirical analysis in china. Appl. Energy 2019, 242, 205–215. 10.1016/j.apenergy.2019.03.067. - DOI
-
- Kraemer F. A.; Palma D.; Braten A. E.; Ammar D. Operationalizing solar energy predictions for sustainable, autonomous iot device management. IEEE Internet of Things J. 2020, 7, 11803–11814. 10.1109/JIOT.2020.3002330. - DOI
-
- Tributsch H. Photo-intercalation: Possible application in solar energy devices. Appl. Phys. 1980, 23, 61–71. 10.1007/BF00899572. - DOI
-
- Paolella A.; Faure C.; Bertoni G.; Marras S.; Guerfi A.; Darwiche A.; Hovington P.; Commarieu B.; Wang Z.; Prato M.; et al. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 2017, 8, 14643.10.1038/ncomms14643. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
