Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review
- PMID: 37969643
- PMCID: PMC10635896
- DOI: 10.1016/j.jaccao.2023.09.002
Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review
Abstract
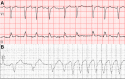
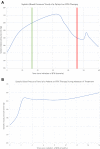
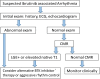
Over the past decade, the treatment landscape of chronic lymphocytic leukemia (CLL) has dramatically changed, shifting from cytotoxic chemotherapy to targeted therapies. Bruton's tyrosine kinase (BTK) inhibitors have revolutionized the treatment of CLL and are increasingly applied in many other malignancies. However, ibrutinib, the first BTK inhibitor approved, is associated with serious toxicities, including atrial fibrillation in up to 38% of patients, ventricular arrhythmias, and other cardiovascular toxicities. Emerging data suggest several newer BTK inhibitors (eg, acalabrutinib, zanubrutinib) are still associated with cardiotoxic risks. This review examines the current state of evidence, including incidence rates, risk factors, mechanisms, and management strategies of cardiovascular toxicities with BTK inhibitors and other CLL therapies. We specifically focus on atrial fibrillation, ventricular arrhythmias/sudden death, hypertension, heart failure, bleeding, and stroke. We also touch on other emerging BTK therapies (eg, pirtobrutinib). Finally, we highlight key unanswered questions and future directions of research.
Keywords: Bruton’s tyrosine kinase inhibitors; acalabrutinib; atrial fibrillation; cardio-oncology; ibrutinib; sudden death; zanubrutinib.
© 2023 Published by Elsevier on behalf of the American College of Cardiology Foundation.
Conflict of interest statement
This work was supported in part by a National Institutes of Health P50-CA140158 grant. Dr Awan has received research funding from Innate Pharma and Pharmacyclics; has provided consulting services to Gilead Sciences, Pharmacyclics, Inc, Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology; and has served on the Speakers Bureau of Abbvie and AstraZeneca. Dr Fradley has received grant support from Medtronic and AstraZeneca; and provides consulting services to Abbvie, AstraZeneca, Johnson and Johnson, Myovant, Pfizer, and Zoll. Dr Kittai is supported by the ASCO Career Development Award; receives research funding from AstraZeneca; and has consulted for AstraZeneca, Abbvie, Beigene, Bristol Myers Squibb, Eli Lilly, Janssen, and KITE. Dr Rogers has received research funding from Genentech, AbbVie, Janssen, and Novartis; has consulted for AstraZeneca, Janssen, Pharmacyclics, AbbVie, Genentech, Beigene, and LOXO@Lilly; and is a scholar in clinical research of the Leukemia and Lymphoma Society (CDP 2331-20). Dr Woyach was supported by R01-CA197870, R01-CA250503, R01-CA177292, R01-CA192928, and scholar in clinical research grants from the Leukemia and Lymphoma Society (CDP 2331-20); has received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys; and has consulted for Janssen and Pharmacyclics. Dr Addison is supported by National Institutes of Health grant numbers K23-HL155890 and R01HL170038, and an American Heart Association-Robert Wood Johnson Foundation Program (Harold Amos) grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

