Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis
- PMID: 37969725
- PMCID: PMC10638506
- DOI: 10.1016/j.apsb.2023.05.025
Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis
Abstract
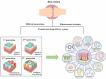
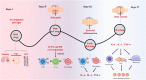
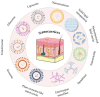
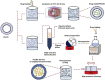
Rheumatoid arthritis is a chronic, systemic autoimmune disease predominantly based on joint lesions with an extremely high disability and deformity rate. Several drugs have been used for the treatment of rheumatoid arthritis, but their use is limited by suboptimal bioavailability, serious adverse effects, and nonnegligible first-pass effects. In contrast, transdermal drug delivery systems (TDDSs) can avoid these drawbacks and improve patient compliance, making them a promising option for the treatment of rheumatoid arthritis (RA). Of course, TDDSs also face unique challenges, as the physiological barrier of the skin makes drug delivery somewhat limited. To overcome this barrier and maximize drug delivery efficiency, TDDSs have evolved in terms of the principle of transdermal facilitation and transdermal facilitation technology, and different generations of TDDSs have been derived, which have significantly improved transdermal efficiency and even achieved individualized controlled drug delivery. In this review, we summarize the different generations of transdermal drug delivery systems, the corresponding transdermal strategies, and their applications in the treatment of RA.
Keywords: Advanced devices; Different generations; Drug therapy; Enhancement strategies; Microneedles; Rheumatoid arthritis; Transdermal delivery mechanism; Transdermal drug delivery systems.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. - PubMed
-
- Qindeel M., Khan D., Ahmed N., Khan S., Rehman A.U. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano. 2020;14:4662–4681. - PubMed
-
- Wang W., Zhou H., Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. - PubMed
-
- Petrelli F., Mariani F.M., Alunno A., Puxeddu I. Pathogenesis of rheumatoid arthritis: one year in review 2022. Clin Exp Rheumatol. 2022;40:475–482. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

